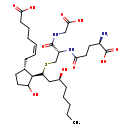| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:59:40 UTC |
|---|
| Update Date | 2020-04-22 18:55:28 UTC |
|---|
| BMDB ID | BMDB0096006 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | S-(11-hydroxy-9-deoxy-delta12-PGD2)-glutathione |
|---|
| Description | S-(11-hydroxy-9-deoxy-delta12-PGD2)-glutathione belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review very few articles have been published on S-(11-hydroxy-9-deoxy-delta12-PGD2)-glutathione. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S-(11-Hydroxy-9-deoxy-δ12-PGD2)-glutathione | Generator | | 9-(S-Glutathionyl)-9-deoxy-delta('12)-prostaglandin D(,2) | HMDB | | (5Z)-7-[(1R,2R)-2-[(3S)-1-{[(2S)-2-{[(4R)-4-amino-4-carboxy-1-hydroxybutylidene]amino}-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl]sulfanyl}-3-hydroxyoctyl]-3-hydroxycyclopentyl]hept-5-enoate | Generator | | (5Z)-7-[(1R,2R)-2-[(3S)-1-{[(2S)-2-{[(4R)-4-amino-4-carboxy-1-hydroxybutylidene]amino}-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl]sulphanyl}-3-hydroxyoctyl]-3-hydroxycyclopentyl]hept-5-enoate | Generator | | (5Z)-7-[(1R,2R)-2-[(3S)-1-{[(2S)-2-{[(4R)-4-amino-4-carboxy-1-hydroxybutylidene]amino}-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl]sulphanyl}-3-hydroxyoctyl]-3-hydroxycyclopentyl]hept-5-enoic acid | Generator |
|
|---|
| Chemical Formula | C30H51N3O10S |
|---|
| Average Molecular Weight | 645.805 |
|---|
| Monoisotopic Molecular Weight | 645.329515557 |
|---|
| IUPAC Name | (5Z)-7-[(1R,2R)-2-[(3S)-1-{[(2S)-2-[(4R)-4-amino-4-carboxybutanamido]-2-[(carboxymethyl)carbamoyl]ethyl]sulfanyl}-3-hydroxyoctyl]-3-hydroxycyclopentyl]hept-5-enoic acid |
|---|
| Traditional Name | (5Z)-7-[(1R,2R)-2-[(3S)-1-{[(2S)-2-[(4R)-4-amino-4-carboxybutanamido]-2-(carboxymethylcarbamoyl)ethyl]sulfanyl}-3-hydroxyoctyl]-3-hydroxycyclopentyl]hept-5-enoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCC[C@H](O)CC(SC[C@@H](NC(=O)CC[C@@H](N)C(O)=O)C(=O)NCC(O)=O)[C@H]1C(O)CC[C@@H]1C\C=C/CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H51N3O10S/c1-2-3-6-10-20(34)16-24(28-19(12-14-23(28)35)9-7-4-5-8-11-26(37)38)44-18-22(29(41)32-17-27(39)40)33-25(36)15-13-21(31)30(42)43/h4,7,19-24,28,34-35H,2-3,5-6,8-18,31H2,1H3,(H,32,41)(H,33,36)(H,37,38)(H,39,40)(H,42,43)/b7-4-/t19-,20-,21+,22+,23?,24?,28+/m0/s1 |
|---|
| InChI Key | BVYNXGKKWADCHD-RXULBTIMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Glutamine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- D-alpha-amino acid
- Tricarboxylic acid or derivatives
- N-acyl-amine
- Fatty acyl
- Fatty amide
- Cyclopentanol
- Cyclic alcohol
- Secondary alcohol
- Amino acid
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Dialkylthioether
- Carboxylic acid
- Sulfenyl compound
- Thioether
- Primary amine
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organosulfur compound
- Primary aliphatic amine
- Organooxygen compound
- Organonitrogen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uml-3101196000-239040b7264c684e98f9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_16) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03gi-0001149000-1acae7aa8f82a5c8458d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01u1-6303794000-25091caeff1adee3d129 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9204440000-c8f07eefb48424253392 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kkc-0015039000-95082e6f257b7828c9bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0019011000-6991fabe9dcf07a53a7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kfw-2913000000-e8d619fbeeb87fe385db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0002019000-9ecc603d1cfdb836ff1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01rt-5202569000-ba96050f00ba151a2ba4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9200310000-375eee33f2aaa98e99f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-0010009000-20714ff89fb377be5481 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01rf-2932044000-05b014894bbe87d0322a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9601000000-4b439af9e2e9d9cd049a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|