| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 16:59:48 UTC |
|---|
| Update Date | 2020-04-22 18:55:31 UTC |
|---|
| BMDB ID | BMDB0096015 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Salsoline-1-carboxylate |
|---|
| Description | Salsoline-1-carboxylate belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. Based on a literature review very few articles have been published on Salsoline-1-carboxylate. |
|---|
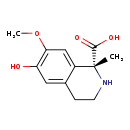
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Salsoline-1-carboxylic acid | Generator | | 1-Methyl-6-hydroxy-7-methoxy-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid | HMDB | | SLN-1C | HMDB | | (1R)-6-Hydroxy-7-methoxy-1-methyl-1,2,3,4-tetrahydroisoquinoline-1-carboxylate | Generator |
|
|---|
| Chemical Formula | C12H15NO4 |
|---|
| Average Molecular Weight | 237.2518 |
|---|
| Monoisotopic Molecular Weight | 237.100107973 |
|---|
| IUPAC Name | (1R)-6-hydroxy-7-methoxy-1-methyl-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid |
|---|
| Traditional Name | (1R)-6-hydroxy-7-methoxy-1-methyl-3,4-dihydro-2H-isoquinoline-1-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=C(O)C=C2CCN[C@@](C)(C(O)=O)C2=C1 |
|---|
| InChI Identifier | InChI=1S/C12H15NO4/c1-12(11(15)16)8-6-10(17-2)9(14)5-7(8)3-4-13-12/h5-6,13-14H,3-4H2,1-2H3,(H,15,16)/t12-/m1/s1 |
|---|
| InChI Key | CJEFWISJWQNPSZ-GFCCVEGCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Alpha-amino acid or derivatives
- Tetrahydroisoquinoline
- Anisole
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Amino acid or derivatives
- Carboxylic acid salt
- Amino acid
- Monocarboxylic acid or derivatives
- Ether
- Secondary amine
- Carboxylic acid
- Secondary aliphatic amine
- Carboxylic acid derivative
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic zwitterion
- Organic salt
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-0900000000-8c59ee94927b59036d93 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03xs-5292000000-49da69ce59df6e06d2c7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-0890000000-411f80ad35ae43b60e5d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0930000000-caaffd2729b5ebeaff9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-0900000000-179c45ccd8d34528a89e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0390000000-b28d4c0c2ca3ac1b765e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000l-0790000000-b0800aefd0966c79a5af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900000000-f41c48731138a98b065b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-fd4f05a9801e9680e5ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02bf-0920000000-8746f0662c242e879a04 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0g6r-0930000000-8cd2ff4ced32cf233b78 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-76e15eb93f3d86f517ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01w4-0920000000-fc4458f3284b44230768 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0buc-4900000000-81ce5983df3383db8d48 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|