| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:00:58 UTC |
|---|
| Update Date | 2020-04-22 18:55:58 UTC |
|---|
| BMDB ID | BMDB0096086 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
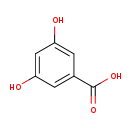
| Common Name | 3,5-Dihydroxybenzoic acid |
|---|
| Description | 3,5-Dihydroxybenzoic acid, also known as alpha-resorcylic acid or a-resorcylate, belongs to the class of organic compounds known as hydroxybenzoic acid derivatives. Hydroxybenzoic acid derivatives are compounds containing a hydroxybenzoic acid (or a derivative), which is a benzene ring bearing a carboxyl and a hydroxyl groups. Based on a literature review a significant number of articles have been published on 3,5-Dihydroxybenzoic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-Resorcylic acid | ChEBI | | a-Resorcylate | Generator | | a-Resorcylic acid | Generator | | alpha-Resorcylate | Generator | | Α-resorcylate | Generator | | Α-resorcylic acid | Generator | | 3,5-Dihydroxybenzoate | Generator | | 3,5-Dihydroxy-benzoic acid | HMDB | | 3,5-Dihydroxybenzoic acid (acd/name 4.0) | HMDB | | 5-Carboxyresorcinol | HMDB | | Benzoate | HMDB | | Benzoic acid | HMDB | | alpha-Resorcylic acid, copper (2+) salt | MeSH, HMDB | | alpha-Resorcylic acid, sodium salt | MeSH, HMDB | | 3,5-DHBA | HMDB | | 3,5-Dihydroxybenzoic acid | HMDB |
|
|---|
| Chemical Formula | C7H6O4 |
|---|
| Average Molecular Weight | 154.121 |
|---|
| Monoisotopic Molecular Weight | 154.026608673 |
|---|
| IUPAC Name | 3,5-dihydroxybenzoic acid |
|---|
| Traditional Name | 3,5-dihydroxybenzoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)C1=CC(O)=CC(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C7H6O4/c8-5-1-4(7(10)11)2-6(9)3-5/h1-3,8-9H,(H,10,11) |
|---|
| InChI Key | UYEMGAFJOZZIFP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxybenzoic acid derivatives. Hydroxybenzoic acid derivatives are compounds containing a hydroxybenzoic acid (or a derivative), which is a benzene ring bearing a carboxyl and a hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Hydroxybenzoic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxybenzoic acid
- Benzoic acid
- Resorcinol
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-2900000000-46dcc0b7ed6c27ee5f7c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-05ai-4092000000-a4387f9715089c352d97 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - CE-QTOF-MS system (Agilent 7100 CE + 6550 QTOF) 15V, Negative | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0019-9000000000-8b567108e3513c709ebd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9300000000-7f42a86a9537534fc938 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1900000000-44e9110e4a5fb09d17c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-004ea53dae9593c251b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-8314761384814f791220 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9700000000-9ee7d6c075af3dc0245c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-0fd6d253983c87b951d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-0900000000-fceb71e190d0e9b6a22a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7900000000-3f3552574ef874889467 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bti-0900000000-74eac3492e2dc880b739 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-1900000000-e54b4355f1c22f9d7ba6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-9100000000-e2fcd97e4e6a1d18054a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0900000000-8723f5ec68dd69c226c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-83aa7ada53ca7119e12b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-d56c533cac680e72de25 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|