| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:00:59 UTC |
|---|
| Update Date | 2020-04-22 18:55:58 UTC |
|---|
| BMDB ID | BMDB0096087 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Peonidin-3-glucoside |
|---|
| Description | Peonidin-3-glucoside, also known as oxycoccicyanin, belongs to the class of organic compounds known as anthocyanidin-3-o-glycosides. These are phenolic compounds containing one anthocyanidin moiety which is O-glycosidically linked to a carbohydrate moiety at the C3-position. Peonidin-3-glucoside exists in all eukaryotes, ranging from yeast to plants to humans. Based on a literature review a small amount of articles have been published on Peonidin-3-glucoside. |
|---|
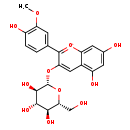
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-O-Methylcyanidin 3-O-beta-D-glucoside | ChEBI | | Oxycoccicyanin | ChEBI | | Peonidin 3-O-glucoside | ChEBI | | 3'-O-Methylcyanidin 3-O-b-D-glucoside | Generator | | 3'-O-Methylcyanidin 3-O-β-D-glucoside | Generator | | Peonidin 3-O-beta-D-glucoside | ChEBI | | Peonidin 3-O-b-D-glucoside | Generator | | Peonidin 3-O-β-D-glucoside | Generator | | Peonidin 3-glucoside | MeSH, HMDB | | Peonidin-3-glucoside | ChEBI | | Peonidin-3-O-glucoside | MeSH |
|
|---|
| Chemical Formula | C22H23O11 |
|---|
| Average Molecular Weight | 463.4114 |
|---|
| Monoisotopic Molecular Weight | 463.124036578 |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1lambda4-chromen-1-ylium |
|---|
| Traditional Name | 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1lambda4-chromen-1-ylium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC1=C(O)C=CC(=C1)C1=[O+]C2=CC(O)=CC(O)=C2C=C1O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C22H22O11/c1-30-15-4-9(2-3-12(15)25)21-16(7-11-13(26)5-10(24)6-14(11)31-21)32-22-20(29)19(28)18(27)17(8-23)33-22/h2-7,17-20,22-23,27-29H,8H2,1H3,(H2-,24,25,26)/p+1/t17-,18-,19+,20-,22-/m1/s1 |
|---|
| InChI Key | ZZWPMFROUHHAKY-OUUKCGNVSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthocyanidin-3-o-glycosides. These are phenolic compounds containing one anthocyanidin moiety which is O-glycosidically linked to a carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Anthocyanidin-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthocyanidin-3-o-glycoside
- Flavonoid-3-o-glycoside
- 3p-methoxyflavonoid-skeleton
- 7-hydroxyflavonoid
- Hydroxyflavonoid
- 5-hydroxyflavonoid
- 4'-hydroxyflavonoid
- Anthocyanidin
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Methoxyphenol
- 1-benzopyran
- Benzopyran
- Methoxybenzene
- Phenoxy compound
- Phenol ether
- Anisole
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Heteroaromatic compound
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Ether
- Oxacycle
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Primary alcohol
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-9203500000-46d99ce5e8628cbe9157 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-03di-2331029000-917c43b617d07d6f073b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0002-0090100000-0807279ecd58dbe38f4e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udi-0039100000-cbeb7a2ec27315d1575a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0100900000-c6004bf80dd72ad2bd7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1300900000-cfe5d43e5a5ed6973d3f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-8911000000-c56dc49b6c675b18b7bd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2300900000-9e7364bc1ea3ed714e5a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-6700900000-759ae58a8bfcf0669b11 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9300000000-c97a6a71b4c020768f6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-484bf4d661bc90952ec8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0039200000-2f06e38c2f05d59c8b3b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w2d-6195100000-e9b66b1890c40defd541 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|