| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:05 UTC |
|---|
| Update Date | 2020-04-22 18:56:24 UTC |
|---|
| BMDB ID | BMDB0096154 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
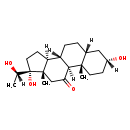
| Common Name | Pregnanetriolone |
|---|
| Description | Pregnanetriolone belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. Based on a literature review a significant number of articles have been published on Pregnanetriolone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha,5beta,20S)-3,17,20-Trihydroxypregnan-11-one | Kegg | | (3a,5b,20S)-3,17,20-Trihydroxypregnan-11-one | Generator | | (3Α,5β,20S)-3,17,20-trihydroxypregnan-11-one | Generator | | 3,17,20-Trihydroxypregnan-11-one (acd/name 4.0) | HMDB | | 5alpha,17alpha,20alpha-Triol-11-one | HMDB | | 5beta-Pregnane-3alpha,17alpha,20alpha-triol-11-one | HMDB | | 11-Keto-pregnanetriol | HMDB | | 3 alpha,17 alpha,20 alpha-Trihydroxy-5 beta-pregnan-11-one | HMDB | | 5b-Pregnane-3a,17a,20a-triol-11-one | HMDB | | 5Β-pregnane-3α,17α,20α-triol-11-one | HMDB | | Pregnanetriolone | KEGG |
|
|---|
| Chemical Formula | C21H34O4 |
|---|
| Average Molecular Weight | 350.4923 |
|---|
| Monoisotopic Molecular Weight | 350.245709576 |
|---|
| IUPAC Name | (1S,2S,5R,7R,10S,11S,14R,15S)-5,14-dihydroxy-14-[(1S)-1-hydroxyethyl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-17-one |
|---|
| Traditional Name | (1S,2S,5R,7R,10S,11S,14R,15S)-5,14-dihydroxy-14-[(1S)-1-hydroxyethyl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-17-one |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](C)(O)[C@@]1(O)CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@]([H])(O)CC[C@]4(C)[C@@]3([H])C(=O)C[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H34O4/c1-12(22)21(25)9-7-16-15-5-4-13-10-14(23)6-8-19(13,2)18(15)17(24)11-20(16,21)3/h12-16,18,22-23,25H,4-11H2,1-3H3/t12-,13+,14+,15-,16-,18+,19-,20-,21-/m0/s1 |
|---|
| InChI Key | WKFXHNDWEHDGQD-ZQRGSSBZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-hydroxysteroid
- 3-hydroxysteroid
- 17-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 11-oxosteroid
- Oxosteroid
- Cyclic alcohol
- Tertiary alcohol
- Ketone
- Secondary alcohol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-074r-1349000000-c5de5f2b7796688751ef | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0udi-2221590000-90db4dbcbd3c9faa403d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0009000000-678c0913e41fc2dcf2f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0039000000-d0619437c3456b1a1f41 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-4298000000-4fd10e13feb314565a72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-238f793de88fab7f70d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053b-0039000000-db756e3e9cc126e68375 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0550-0096000000-53afad53a5f5f4778b88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-2c75cdbeee7a59d1557b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000b-0039000000-e823529302be730044a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0097000000-853e779825019bff5230 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-0009000000-8a455e1c9f1ef9c64216 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03yi-0978000000-a845c764af3584615ac4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-2900000000-c38c98aa4785dba03cab | View in MoNA |
|---|
|
|---|