| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:06 UTC |
|---|
| Update Date | 2020-04-22 18:56:24 UTC |
|---|
| BMDB ID | BMDB0096155 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | S-Phenylmercapturic acid |
|---|
| Description | S-Phenylmercapturic acid, also known as S-phenylmercaptate, belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. Based on a literature review very few articles have been published on S-Phenylmercapturic acid. |
|---|
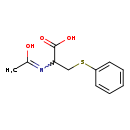
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S-Phenylmercaptate | Generator | | S-Phenylmercaptic acid | Generator | | S-Phenyl-N-acetylcysteine | MeSH, HMDB | | S-Phenyl-N-acetylcysteine, (DL)-isomer | MeSH, HMDB | | Phenylmercapturic acid | MeSH, HMDB | | 2-acetamido-3-Phenylthiopropanoic acid | MeSH, HMDB | | 2-[(1-Hydroxyethylidene)amino]-3-(phenylsulfanyl)propanoate | Generator, HMDB | | 2-[(1-Hydroxyethylidene)amino]-3-(phenylsulphanyl)propanoate | Generator, HMDB | | 2-[(1-Hydroxyethylidene)amino]-3-(phenylsulphanyl)propanoic acid | Generator, HMDB | | S-Phenylmercapturic acid | MeSH |
|
|---|
| Chemical Formula | C11H13NO3S |
|---|
| Average Molecular Weight | 239.291 |
|---|
| Monoisotopic Molecular Weight | 239.061613977 |
|---|
| IUPAC Name | 2-[(1-hydroxyethylidene)amino]-3-(phenylsulfanyl)propanoic acid |
|---|
| Traditional Name | 2-[(1-hydroxyethylidene)amino]-3-(phenylsulfanyl)propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(O)=NC(CSC1=CC=CC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H13NO3S/c1-8(13)12-10(11(14)15)7-16-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15) |
|---|
| InChI Key | CICOZWHZVMOPJS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Cysteine or derivatives
- Aryl thioether
- Thiophenol ether
- Alkylarylthioether
- Monocyclic benzene moiety
- Benzenoid
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Thioether
- Sulfenyl compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9810000000-ed7dc9c7543668c35682 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-02p3-9333000000-97be0730bd885288c58c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0980000000-b921bf6a20ea43c582da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ikd-0910000000-6c9f36e99b97449968b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02hi-9700000000-bfa9760500e81217cac2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-0790000000-fbc303613619be6d0b18 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-bb17ac9a6848d4ff050c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-8900000000-3677213c11e55480ecba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0950000000-73936d02c6fc457f888b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-2900000000-2cf91ee3bc41503759f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06vi-5900000000-7ab6fd1ccd14bac03f95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-3900000000-c7e4ebb011fab224d5aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-9604afb1cae23bc5502c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2900000000-fd1806c53455483908e9 | View in MoNA |
|---|
|
|---|