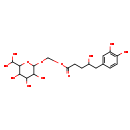| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:21 UTC |
|---|
| Update Date | 2020-05-11 20:27:34 UTC |
|---|
| BMDB ID | BMDB0096170 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Hydroxy-5-(3',4'-dihydroxyphenyl)-valeric acid-O-methyl-O-glucuronide |
|---|
| Description | 4-Hydroxy-5-(3',4'-dihydroxyphenyl)-valeric acid-O-methyl-O-glucuronide belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. 4-Hydroxy-5-(3',4'-dihydroxyphenyl)-valeric acid-O-methyl-O-glucuronide is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-5-(3',4'-dihydroxyphenyl)-valerate-O-methyl-O-glucuronide | Generator | | {[6-(dihydroxymethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}methyl 5-(3,4-dihydroxyphenyl)-4-hydroxypentanoic acid | Generator |
|
|---|
| Chemical Formula | C18H26O12 |
|---|
| Average Molecular Weight | 434.3918 |
|---|
| Monoisotopic Molecular Weight | 434.142426296 |
|---|
| IUPAC Name | {[6-(dihydroxymethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}methyl 5-(3,4-dihydroxyphenyl)-4-hydroxypentanoate |
|---|
| Traditional Name | {[6-(dihydroxymethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}methyl 5-(3,4-dihydroxyphenyl)-4-hydroxypentanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(CCC(=O)OCOC1OC(C(O)O)C(O)C(O)C1O)CC1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C18H26O12/c19-9(5-8-1-3-10(20)11(21)6-8)2-4-12(22)28-7-29-18-15(25)13(23)14(24)16(30-18)17(26)27/h1,3,6,9,13-21,23-27H,2,4-5,7H2 |
|---|
| InChI Key | KRZPFELARLSRKT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-glycosyl compound
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Fatty acid ester
- Phenol
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Monosaccharide
- Oxane
- Carboxylic acid ester
- Secondary alcohol
- Acetal
- Carbonyl hydrate
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00r2-9823300000-5346a972bb5ef897bb80 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0fa6-3592115000-2d8582272b3be0f7b412 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0590200000-750b3651fe12a5cc5d07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2890100000-f3bf4d6327896631f04c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-4930000000-598c069c5f10c48712c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4r-1291200000-f723c8ccf2c013c48383 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a71-3891100000-2f86046cd68f778001ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9770000000-552c08fe92653d5ebf5c | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|