| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:24 UTC |
|---|
| Update Date | 2020-04-22 18:56:31 UTC |
|---|
| BMDB ID | BMDB0096173 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
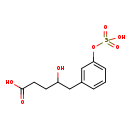
| Common Name | 4-Hydroxy-5-(3'-hydroxyphenyl)-valeric acid-3'-O-sulphate |
|---|
| Description | 4-Hydroxy-5-(3'-hydroxyphenyl)-valeric acid-3'-O-sulphate belongs to the class of organic compounds known as sulfated fatty acids. These are fatty acids containing linked to a sulfate group linked to its tail. 4-Hydroxy-5-(3'-hydroxyphenyl)-valeric acid-3'-O-sulphate is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-5-(3'-hydroxyphenyl)-valerate-3'-O-sulfate | Generator | | 4-Hydroxy-5-(3'-hydroxyphenyl)-valerate-3'-O-sulphate | Generator | | 4-Hydroxy-5-(3'-hydroxyphenyl)-valeric acid-3'-O-sulfuric acid | Generator | | 4-Hydroxy-5-(3'-hydroxyphenyl)-valeric acid-3'-O-sulphuric acid | Generator |
|
|---|
| Chemical Formula | C11H14O7S |
|---|
| Average Molecular Weight | 290.29 |
|---|
| Monoisotopic Molecular Weight | 290.046023492 |
|---|
| IUPAC Name | 4-hydroxy-5-[3-(sulfooxy)phenyl]pentanoic acid |
|---|
| Traditional Name | 4-hydroxy-5-[3-(sulfooxy)phenyl]pentanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(CCC(O)=O)CC1=CC(OS(O)(=O)=O)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C11H14O7S/c12-9(4-5-11(13)14)6-8-2-1-3-10(7-8)18-19(15,16)17/h1-3,7,9,12H,4-6H2,(H,13,14)(H,15,16,17) |
|---|
| InChI Key | DMBFBGLRQKIWGT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfated fatty acids. These are fatty acids containing linked to a sulfate group linked to its tail. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Sulfated fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Sulfated fatty acid
- Arylsulfate
- Medium-chain hydroxy acid
- Phenoxy compound
- Medium-chain fatty acid
- Hydroxy fatty acid
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Organic sulfuric acid or derivatives
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-2930000000-c31530e66030c284f01a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-7639100000-0bb9f6f96ca7fe28cc49 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-a2b5f7060b0df3226fba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abc-3690000000-822d26875ce7b46b0550 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02vr-9610000000-34259559781de7ec7449 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e41e1fcfcff2d5e5d761 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-059f-1690000000-a4b8c1ecfab37d28870c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9600000000-6d9ef595bb614d5fc3c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0290000000-dd710fd8dbe5a48e9de4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pdi-1970000000-9d5588eae01cd7a34efb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-5910000000-d60a62e1761a1b9d2f48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-61dd1e3752cfa2914fa5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-4960000000-2c73108735eabb264c40 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9200000000-2159b80a3aa5ea0a9f11 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|