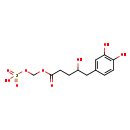| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:26 UTC |
|---|
| Update Date | 2020-04-22 18:56:32 UTC |
|---|
| BMDB ID | BMDB0096175 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-methyl-O-sulphate |
|---|
| Description | 4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-methyl-O-sulphate belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. 4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-methyl-O-sulphate is an extremely weak basic (essentially neutral) compound (based on its pKa). These are fatty acids in which the chain bears an hydroxyl group. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-5-(dihydroxyphenyl)-valerate-O-methyl-O-sulfate | Generator | | 4-Hydroxy-5-(dihydroxyphenyl)-valerate-O-methyl-O-sulphate | Generator | | 4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-methyl-O-sulfuric acid | Generator | | 4-Hydroxy-5-(dihydroxyphenyl)-valeric acid-O-methyl-O-sulphuric acid | Generator | | ({[5-(3,4-dihydroxyphenyl)-4-hydroxypentanoyl]oxy}methoxy)sulfonate | Generator | | ({[5-(3,4-dihydroxyphenyl)-4-hydroxypentanoyl]oxy}methoxy)sulphonate | Generator | | ({[5-(3,4-dihydroxyphenyl)-4-hydroxypentanoyl]oxy}methoxy)sulphonic acid | Generator |
|
|---|
| Chemical Formula | C12H16O9S |
|---|
| Average Molecular Weight | 336.315 |
|---|
| Monoisotopic Molecular Weight | 336.0515028 |
|---|
| IUPAC Name | ({[5-(3,4-dihydroxyphenyl)-4-hydroxypentanoyl]oxy}methoxy)sulfonic acid |
|---|
| Traditional Name | {[5-(3,4-dihydroxyphenyl)-4-hydroxypentanoyl]oxy}methoxysulfonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(CCC(=O)OCOS(O)(=O)=O)CC1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C12H16O9S/c13-9(5-8-1-3-10(14)11(15)6-8)2-4-12(16)20-7-21-22(17,18)19/h1,3,6,9,13-15H,2,4-5,7H2,(H,17,18,19) |
|---|
| InChI Key | YDBDCESYCNBVOY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenediols |
|---|
| Direct Parent | Catechols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Fatty acid ester
- Monocyclic benzene moiety
- Fatty acyl
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Organic sulfuric acid or derivatives
- Carboxylic acid ester
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-1910000000-4a5c09f7b3ee883b2d5e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-03di-5839730000-1531d8c1ee9c416206a8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-1594000000-86a2c9230f55bff07933 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08gl-2921000000-6e952fb08ecd821cd0ef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9830000000-5498b34f6193d780b484 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-1395000000-347ecd10983a6d79f9a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0570-2591000000-c3c45a073daac381396f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-9310000000-c97e753cffdcf5cf2fe6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-1896000000-8b39607bf10b567429c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1910000000-84068f333ec1dd914e1f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-1910000000-3d4972cb505afd7dacc5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a70-0194000000-3482d8092902a0849865 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9110000000-8b9d7a164b4ad82e5ea8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9500000000-62190f1410c7194d1418 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|