| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:31 UTC |
|---|
| Update Date | 2020-04-22 18:56:33 UTC |
|---|
| BMDB ID | BMDB0096180 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-hydroxybenzoic acid-4-O-sulphate |
|---|
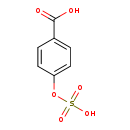
| Description | 4-hydroxybenzoic acid-4-O-sulphate belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. 4-hydroxybenzoic acid-4-O-sulphate is an extremely strong acidic compound (based on its pKa). It also inhibits protein isoprenylation, depletes plasma glutamine, increases production of foetal haemoglobin through transcriptional activation of the γ-globin gene and affects hPPARγ activation. It inhibits cell proliferation, invasion and migration and induces apoptosis in glioma cells. 4-phenylbutyric acid is a monocarboxylic acid the structure of which is that of butyric acid substituted with a phenyl group at C-4. It is a histone deacetylase inhibitor that displays anticancer activity. 4-hydroxybenzoic acid-4-O-sulphate is a conjugate of 4-hydroxybenzoic acid and sulphate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxybenzoate-4-O-sulfate | Generator | | 4-Hydroxybenzoate-4-O-sulphate | Generator | | 4-Hydroxybenzoic acid-4-O-sulfuric acid | Generator | | 4-Hydroxybenzoic acid-4-O-sulphuric acid | Generator | | 4-(Sulfooxy)benzoate | Generator | | 4-(Sulphooxy)benzoate | Generator | | 4-(Sulphooxy)benzoic acid | Generator | | 4-Hydroxybenzoate 4-O-sulfate | Generator | | 4-Hydroxybenzoate 4-O-sulphate | Generator | | 4-Hydroxybenzoic acid 4-O-sulfuric acid | Generator | | 4-Hydroxybenzoic acid 4-O-sulphuric acid | Generator |
|
|---|
| Chemical Formula | C7H6O6S |
|---|
| Average Molecular Weight | 218.184 |
|---|
| Monoisotopic Molecular Weight | 217.988508614 |
|---|
| IUPAC Name | 4-(sulfooxy)benzoic acid |
|---|
| Traditional Name | 4-(sulfooxy)benzoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)C1=CC=C(OS(O)(=O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C7H6O6S/c8-7(9)5-1-3-6(4-2-5)13-14(10,11)12/h1-4H,(H,8,9)(H,10,11,12) |
|---|
| InChI Key | RJTYSXVYCZAUHE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Benzoic acid or derivatives
- Benzoic acid
- Phenoxy compound
- Benzoyl
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Monocyclic benzene moiety
- Benzenoid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00y0-3930000000-4cafa70d8f92aa8d2e7b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fk9-8490000000-00dca0f4a33122436c6d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-014r-1890000000-21971529a937750ab646 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0006-9200000000-4c031637db4f8f5af9d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0290000000-22f965679e6ee4eec6fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0960000000-ba412848c4ed4bb4f103 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9100000000-90b89c265513a3aba1fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0490000000-72571253054ef2548070 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00rl-3920000000-6fd9b49382c4a983a7f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9600000000-4d293a0ed6674e1822d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-425b35e8af5f26b0cda9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0390000000-a7c5464a902a4f729940 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-7900000000-acb31aa2cbcfb141cf2c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-ff32c1b87d0a3d4d9ee2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0910000000-1005ff47ab97345013f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fdn-9400000000-17bc7a2fad3ce3238d66 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|