| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:37 UTC |
|---|
| Update Date | 2020-05-11 20:27:40 UTC |
|---|
| BMDB ID | BMDB0096187 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
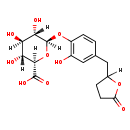
| Common Name | 5-(3',4'-Dihydroxyphenyl)-gamma-valerolactone-4'-O-glucuronide |
|---|
| Description | 5-(3',4'-Dihydroxyphenyl)-gamma-valerolactone-4'-O-glucuronide belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. 5-(3',4'-Dihydroxyphenyl)-gamma-valerolactone-4'-O-glucuronide is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-(3',4'-Dihydroxyphenyl)-g-valerolactone-4'-O-glucuronide | Generator | | 5-(3',4'-Dihydroxyphenyl)-γ-valerolactone-4'-O-glucuronide | Generator |
|
|---|
| Chemical Formula | C17H20O10 |
|---|
| Average Molecular Weight | 384.3347 |
|---|
| Monoisotopic Molecular Weight | 384.10564686 |
|---|
| IUPAC Name | (2R,3R,4R,5S,6R)-3,4,5-trihydroxy-6-{2-hydroxy-4-[(5-oxooxolan-2-yl)methyl]phenoxy}oxane-2-carboxylic acid |
|---|
| Traditional Name | (2R,3R,4R,5S,6R)-3,4,5-trihydroxy-6-{2-hydroxy-4-[(5-oxooxolan-2-yl)methyl]phenoxy}oxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]C1(CC2=CC(O)=C(O[C@@]3([H])O[C@@]([H])(C(O)=O)[C@]([H])(O)[C@@]([H])(O)[C@]3([H])O)C=C2)CCC(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C17H20O10/c18-9-6-7(5-8-2-4-11(19)25-8)1-3-10(9)26-17-14(22)12(20)13(21)15(27-17)16(23)24/h1,3,6,8,12-15,17-18,20-22H,2,4-5H2,(H,23,24)/t8?,12-,13-,14+,15-,17+/m1/s1 |
|---|
| InChI Key | OTBJYBQGMPICIK-GHPVWUPISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Alkyl glycoside
- O-glycosyl compound
- Phenoxy compound
- Phenol ether
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Beta-hydroxy acid
- Phenol
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Fatty acyl
- Gamma butyrolactone
- Hydroxy acid
- Monosaccharide
- Benzenoid
- Oxane
- Pyran
- Tetrahydrofuran
- Lactone
- Secondary alcohol
- Carboxylic acid ester
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid
- Acetal
- Carboxylic acid derivative
- Polyol
- Organic oxide
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-07vs-7393000000-9e5d2516c1bfcf90a3a4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0a4i-3022029000-4fb28db4992e513764ac | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ap0-0449000000-fe5b377019be11eb4318 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0931000000-e2aac4e8d4e763754c3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aba-1910000000-f04fb36a8d821e654fa4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0540-1339000000-777b495cd5dadc619ff3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4895000000-511a2a454fae1b223a24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9420000000-274ad576dfc356cebb55 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|