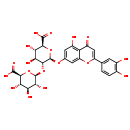| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:14 UTC |
|---|
| Update Date | 2020-05-11 20:27:58 UTC |
|---|
| BMDB ID | BMDB0096224 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Luteolin 7-O-[beta-D-glucuronosyl-(1->2)-beta-D-glucuronide] |
|---|
| Description | Luteolin 7-O-[beta-D-glucuronosyl-(1->2)-beta-D-glucuronide], also known as luteolin 7-O-beta-D-diglucuronide, belongs to the class of organic compounds known as benzonitriles. These are organic compounds containing a benzene bearing a nitrile substituent. These are compounds containing a carbohydrate moiety which is o-glycosidically linked to one of the flavonoid backbones (2-phenylchromen-4-one, 3-phenylchromen-4-one or 4-phenylcoumarin). Luteolin 7-O-[beta-D-glucuronosyl-(1->2)-beta-D-glucuronide] is an extremely weak basic (essentially neutral) compound (based on its pKa). Luteolin 7-O-[beta-D-glucuronosyl-(1->2)-beta-D-glucuronide] exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Luteolin 7-O-beta-D-diglucuronide | ChEBI | | Luteolin-7-O-[beta-glucuronosyl-(1->2)-beta-glucuronide] | ChEBI | | Luteolin 7-O-b-D-diglucuronide | Generator | | Luteolin 7-O-β-D-diglucuronide | Generator | | Luteolin-7-O-[b-glucuronosyl-(1->2)-b-glucuronide] | Generator | | Luteolin-7-O-[β-glucuronosyl-(1->2)-β-glucuronide] | Generator | | Luteolin 7-O-[b-D-glucuronosyl-(1->2)-b-D-glucuronide] | Generator | | Luteolin 7-O-[β-D-glucuronosyl-(1->2)-β-D-glucuronide] | Generator | | Luteolin-7-O-[b-D-glucuronosyl-(1->2)-b-D-glucuronide] | HMDB | | Luteolin-7-O-[β-D-glucuronosyl-(1->2)-β-D-glucuronide] | HMDB |
|
|---|
| Chemical Formula | C27H26O18 |
|---|
| Average Molecular Weight | 638.4845 |
|---|
| Monoisotopic Molecular Weight | 638.111914028 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(2S,3R,4S,5S,6S)-6-carboxy-2-{[2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen-7-yl]oxy}-4,5-dihydroxyoxan-3-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-6-{[(2S,3R,4S,5S,6S)-6-carboxy-2-{[2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxochromen-7-yl]oxy}-4,5-dihydroxyoxan-3-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@H](O[C@H]2OC2=CC(O)=C3C(=O)C=C(OC3=C2)C2=CC(O)=C(O)C=C2)C(O)=O)O[C@@H]([C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C27H26O18/c28-9-2-1-7(3-10(9)29)13-6-12(31)15-11(30)4-8(5-14(15)42-13)41-27-23(19(35)18(34)22(44-27)25(39)40)45-26-20(36)16(32)17(33)21(43-26)24(37)38/h1-6,16-23,26-30,32-36H,(H,37,38)(H,39,40)/t16-,17-,18-,19-,20+,21-,22-,23+,26-,27+/m0/s1 |
|---|
| InChI Key | PBBVWJQPAZYQDB-DBFWEQBMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzonitriles. These are organic compounds containing a benzene bearing a nitrile substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzonitriles |
|---|
| Direct Parent | Benzonitriles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzonitrile
- M-xylene
- Xylene
- Styrene
- Aniline or substituted anilines
- Aminopyrimidine
- Hydropyrimidine
- Pyrimidine
- Imidolactam
- Heteroaromatic compound
- Azacycle
- Secondary amine
- Nitrile
- Carbonitrile
- Organoheterocyclic compound
- Amine
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fk9-3230092000-ea586362caa9811d734a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-007a-0190708000-3d49ae4f96ee951e9a47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0190300000-58045aa21bff6be8fde8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0590100000-3a3f84a9e4792f8e9f1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-2683988000-404d4bc0c3bffae7c491 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2691521000-3e459376349ced77a414 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000m-3890210000-d1d3d2eefee06ac61d1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090004000-80a4e61137f91cc1999a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0090000000-ad6070afb384abda8f3b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0090000000-ad6070afb384abda8f3b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090006000-82b8470e9ac8715f73b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0020-0090000000-c3ca49aca7adc52662ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0020-0090000000-68306dd5c050e9a6b46b | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|