| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:17 UTC |
|---|
| Update Date | 2020-04-22 18:56:51 UTC |
|---|
| BMDB ID | BMDB0096227 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | heparan sulfate alpha-D-glucosaminide |
|---|
| Description | heparan sulfate alpha-D-glucosaminide belongs to the class of organic compounds known as oligosaccharide sulfates. These are carbohydrates containing between 3 and 9 sugar units, one of which bear one or more sulfate groups. heparan sulfate alpha-D-glucosaminide is a very strong basic compound (based on its pKa). These are trisaccharides containing three hexose carbohydrates. |
|---|
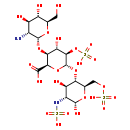
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Heparan sulfate a-D-glucosaminide | Generator | | Heparan sulfate α-D-glucosaminide | Generator | | Heparan sulfuric acid a-D-glucosaminide | Generator | | Heparan sulfuric acid alpha-D-glucosaminide | Generator | | Heparan sulfuric acid α-D-glucosaminide | Generator | | Heparan sulphate a-D-glucosaminide | Generator | | Heparan sulphate alpha-D-glucosaminide | Generator | | Heparan sulphate α-D-glucosaminide | Generator | | Heparan sulphuric acid a-D-glucosaminide | Generator | | Heparan sulphuric acid alpha-D-glucosaminide | Generator | | Heparan sulphuric acid α-D-glucosaminide | Generator |
|
|---|
| Chemical Formula | C18H32N2O24S3 |
|---|
| Average Molecular Weight | 756.641 |
|---|
| Monoisotopic Molecular Weight | 756.050712032 |
|---|
| IUPAC Name | (2R,3S,4S,5R,6R)-3-{[(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6S)-4,6-dihydroxy-5-(sulfoamino)-2-[(sulfooxy)methyl]oxan-3-yl]oxy}-4-hydroxy-5-(sulfooxy)oxane-2-carboxylic acid |
|---|
| Traditional Name | (2R,3S,4S,5R,6R)-3-{[(2R,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6S)-4,6-dihydroxy-5-(sulfoamino)-2-[(sulfooxy)methyl]oxan-3-yl]oxy}-4-hydroxy-5-(sulfooxy)oxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](OS(O)(=O)=O)[C@H](O[C@H]2[C@H](O)[C@@H](NS(O)(=O)=O)[C@@H](O)O[C@@H]2COS(O)(=O)=O)O[C@H]1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H32N2O24S3/c19-5-8(23)7(22)3(1-21)40-17(5)42-12-10(25)13(44-47(35,36)37)18(43-14(12)15(26)27)41-11-4(2-38-46(32,33)34)39-16(28)6(9(11)24)20-45(29,30)31/h3-14,16-18,20-25,28H,1-2,19H2,(H,26,27)(H,29,30,31)(H,32,33,34)(H,35,36,37)/t3-,4-,5-,6-,7-,8-,9-,10+,11-,12+,13-,14-,16+,17-,18-/m1/s1 |
|---|
| InChI Key | JAXHHZAWQAGVNS-BCMGMHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharide sulfates. These are carbohydrates containing between 3 and 9 sugar units, one of which bear one or more sulfate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharide sulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide sulfate
- Aminoglycoside core
- Fatty acyl glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Glycosyl compound
- O-glycosyl compound
- Amino saccharide
- Fatty acyl
- Sulfuric acid monoamide
- Oxane
- Pyran
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Alkyl sulfate
- Organic sulfuric acid or derivatives
- Amino acid or derivatives
- Secondary alcohol
- 1,2-aminoalcohol
- Hemiacetal
- Amino acid
- Carboxylic acid derivative
- Acetal
- Organoheterocyclic compound
- Carboxylic acid
- Oxacycle
- Monocarboxylic acid or derivatives
- Carbonyl group
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organonitrogen compound
- Primary amine
- Alcohol
- Amine
- Primary alcohol
- Organic nitrogen compound
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-009i-9501204500-e04de8c667184fe2d9bf | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0334149600-300041618676cb90ebee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0adl-0179176200-1cfa6ee08e19b91a3ea8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4u-1891120000-7cbac70d24eb2a7f2aa6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2200490100-ae22d1d631b129713ec1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-5928057300-357a060dcfa91e5a9877 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002s-9607010000-d4a89f66459b4e70c0c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004r-0000090500-9d437087b0ec29639d48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0574-0910371700-270422249910eb3901ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-5590010100-50280c3893bb1c5fcd01 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000001900-98c878cd737647cbd38f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-4001279300-1278df35ff46abd32f1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zgs-9160146000-890034e540a9725faf0e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|