| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-05-05 15:49:08 UTC |
|---|
| Update Date | 2020-05-05 18:38:56 UTC |
|---|
| BMDB ID | BMDB0109650 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Albendazole |
|---|
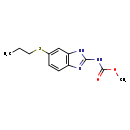
| Description | Albendazole, also known as albenza or SK and F62979, belongs to the class of organic compounds known as 2-benzimidazolylcarbamic acid esters. These are aromatic heteropolycyclic compounds that contain a carbamic acid ester group, which is N-linked to the C2-atom of a benzimidazole moiety. Albendazole is a drug which is used for the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, taenia solium and for the treatment of cystic hydatid disease of the liver, lung, and peritoneum, caused by the larval form of the dog tapeworm, echinococcus granulosus. Albendazole is a strong basic compound (based on its pKa). Albendazole exists in all living organisms, ranging from bacteria to humans. In humans, albendazole is involved in tamoxifen metabolism pathway. Albendazole is a potentially toxic compound. Symptoms of overdose include elevated liver enzymes, headaches, hair loss, low levels of white blood cells (neutropenia), fever, and itching. Albendazole causes degenerative alterations in the tegument and intestinal cells of the worm by diminishing its energy production, ultimately leading to immobilization and death of the parasite. The loss of the cytoplasmic microtubules leads to impaired uptake of glucose by the larval and adult stages of the susceptible parasites, and depletes their glycogen stores. Degenerative changes in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosomes result in decreased production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth. Route of Elimination: Albendazole is rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5-(Propylthio)-1H-benzimidazol-2-yl)carbamic acid methyl ester | ChEBI | | 5-(Propylthio)-2-carbomethoxyaminobenzimidazole | ChEBI | | Albenza | ChEBI | | Eskazole | ChEBI | | O-Methyl N-(5-(propylthio)-2-benzimidazolyl)carbamate | ChEBI | | Proftril | ChEBI | | Valbazen | ChEBI | | Zentel | ChEBI | | (5-(Propylthio)-1H-benzimidazol-2-yl)carbamate methyl ester | Generator | | O-Methyl N-(5-(propylthio)-2-benzimidazolyl)carbamic acid | Generator | | Albendazole armstrong brand | HMDB | | Albendazole diba brand | HMDB | | Albendazole pfizer brand | HMDB | | Albendoral | HMDB | | Armstrong brand OF albendazole | HMDB | | SK And F62979 | HMDB | | Albendazole sanicoopa brand | HMDB | | Albendazole valdecasas brand | HMDB | | Bendapar | HMDB | | Digezanol | HMDB | | Endoplus | HMDB | | Fustery brand OF albendazole | HMDB | | Hormona brand OF albendazole | HMDB | | Liferpal brand OF albendazole | HMDB | | Lurdex | HMDB | | Mediamix V disthelm | HMDB | | Metiazol | HMDB | | Monohydrochloride, albendazole | HMDB | | SK And F-62979 | HMDB | | Sanicoopa brand OF albendazole | HMDB | | Albendazole fustery brand | HMDB | | Gascop | HMDB | | Noé-socopharm brand OF albendazole | HMDB | | Pfizer brand OF albendazole | HMDB | | SmithKline beecham brand OF albendazole | HMDB | | V Disthelm, mediamix | HMDB | | Albendazole hormona brand | HMDB | | Albendazole liferpal brand | HMDB | | Albendazole monohydrochloride | HMDB | | Albendazole noé-socopharm brand | HMDB | | Bilutac | HMDB | | Diba brand OF albendazole | HMDB | | Disthelm | HMDB | | Disthelm, mediamix V | HMDB | | Noé socopharm brand OF albendazole | HMDB | | SK And F 62979 | HMDB | | Valdecasas brand OF albendazole | HMDB | | Andazol | MeSH |
|
|---|
| Chemical Formula | C12H15N3O2S |
|---|
| Average Molecular Weight | 265.331 |
|---|
| Monoisotopic Molecular Weight | 265.088497429 |
|---|
| IUPAC Name | methyl N-[6-(propylsulfanyl)-1H-1,3-benzodiazol-2-yl]carbamate |
|---|
| Traditional Name | albendazol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCSC1=CC2=C(C=C1)N=C(NC(=O)OC)N2 |
|---|
| InChI Identifier | InChI=1S/C12H15N3O2S/c1-3-6-18-8-4-5-9-10(7-8)14-11(13-9)15-12(16)17-2/h4-5,7H,3,6H2,1-2H3,(H2,13,14,15,16) |
|---|
| InChI Key | HXHWSAZORRCQMX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-benzimidazolylcarbamic acid esters. These are aromatic heteropolycyclic compounds that contain a carbamic acid ester group, which is N-linked to the C2-atom of a benzimidazole moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzimidazoles |
|---|
| Sub Class | 2-benzimidazolylcarbamic acid esters |
|---|
| Direct Parent | 2-benzimidazolylcarbamic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-benzimidazolylcarbamic acid ester
- Aryl thioether
- Thiophenol ether
- Alkylarylthioether
- Benzenoid
- Azole
- Imidazole
- Carbamic acid ester
- Heteroaromatic compound
- Carbonic acid derivative
- Thioether
- Azacycle
- Sulfenyl compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0zg0-1469000000-46c2b274ef14c131df22 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00dl-2920000000-8bfa1e52a364c2e1f3ac | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0zg0-1469000000-46c2b274ef14c131df22 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00dl-2920000000-8bfa1e52a364c2e1f3ac | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00vl-3960000000-6405beb97aaf7baeef1e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-01q9-0090000000-672ce5296af7aee8b705 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-001i-0390000000-5714d326ce05257dd82d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000i-0910000000-e659044fe535a4187c4c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000i-0900000000-a1c7141f3f860d69dca2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000i-0900000000-96ae063a35ee4fe53390 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-052r-2900000000-6103a7173b7b7689ee52 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-9600000000-7a4cee3070ee6f08b2cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-9200000000-109010056dd851a50ae4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-9000000000-9f06e0952a63ae1312b3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-001r-0790000000-8ba2b09e2eefe111496f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-001i-0290000000-db0e07ed437380dd7f56 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0006-0910000000-80dd825626a5009e8924 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-052f-0900000000-ea3849a4ab993b24b4f0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0090000000-fe82219d2c01efc3494d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00lr-0090000000-6d1f652e7ecf943d7400 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-001i-0190000000-ab8f5993bbc5ebfcb04c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-000x-0950000000-0c5ca5956802aa312e11 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-0900000000-02e757b669a99db25608 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-052f-0900000000-bef5aa7a328477b1c279 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-1190000000-b3b5e0c1c81c186e0323 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-6390000000-49710f98d2607932e74d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01vo-6920000000-f7a76ee929303af68012 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-06si-5390000000-662c83573e8207d72065 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-5960000000-7fffbfea1f1e67fdbb9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01rx-9610000000-014e3ec45924170b49d0 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100.40 MHz, DMSO-d6, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|