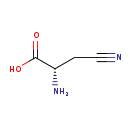
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0006-2910000000-cdae81bdd716faf914cb | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-0910000000-76fcf98a116d8f5fb3a3 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0006-0900000000-87a768329cbe7349b129 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-9000000000-b588e4f0716ef2c3b6ea | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014i-9100000000-84aa6cdc91672d2d0c40 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0002-9000000000-fe247d540e001b5b738b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 35V, negative | splash10-0006-9000000000-0b174b16428577353612 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, negative | splash10-0udi-9000000000-b9d26a1c944b0649cadc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, negative | splash10-0002-9000000000-f17c4c09160c2e81931a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0002-9000000000-c35c2982261090e1ac28 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0002-9000000000-970b9d3c7c142d5bc7ba | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0002-9000000000-1638709f55d082c64835 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-0002-9000000000-cc48714167e1ba803c7c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, negative | splash10-03di-0900000000-45c3feae2e1ceff7377d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, negative | splash10-03di-0900000000-bf090b5f90b37f228ec4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, negative | splash10-03di-1900000000-48b98dfc454b8b5ef5d4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 0V, negative | splash10-03di-2900000000-7490f45dd2e226996a58 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, negative | splash10-03di-4900000000-ba685d69c16b14e76f7f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, negative | splash10-03dj-9800000000-9eb50c4dbae9090478e7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-01ot-9400000000-d239b0a8038ad871ad6b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0002-9200000000-1e9123df57023d142592 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0002-9100000000-bee64b38054305fe23fc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-0002-9000000000-36456509bbb17410a4c0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-0002-9000000000-a9cd3d6dc542f1ebd0b3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 4V, negative | splash10-006t-9000000000-0923f4b6979f216eb757 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, negative | splash10-0002-9000000000-dc0d16ea8ae536cb82eb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, negative | splash10-0udi-9000000000-e742d1778ed2916e7989 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0006-9000000000-46983704961d3c708c40 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, positive | splash10-00di-9000000000-783db8104c08d8983467 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, positive | splash10-0a4j-8900000000-c3a0f4587b5f55cd8cf9 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|