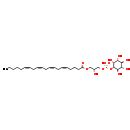| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-05-05 15:49:37 UTC |
|---|
| Update Date | 2020-05-05 18:39:03 UTC |
|---|
| BMDB ID | BMDB0109672 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | LysoPI(20:4(5Z,8Z,11Z,14Z)/0:0) |
|---|
| Description | LysoPI(20:4(5Z,8Z,11Z,14Z)/0:0), also known as pi(20:4(5Z,8Z,11Z,14Z)/0:0) or lysophosphatidylinositol(20:4), belongs to the class of organic compounds known as 1-acyl-sn-glycerol-3-phosphoinositols. These are glycerophosphoinositols where the glycerol is acylated only at position O-1 with a fatty acid. LysoPI(20:4(5Z,8Z,11Z,14Z)/0:0) is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(5Z,8Z,11Z,14Z-Eicosatetraenoyl)-sn-glycero-3-phospho-(1'-myo-inositol) | ChEBI | | 1-Arachidonoylglycerophosphoinositol | ChEBI | | PI(20:4(5Z,8Z,11Z,14Z)/0:0) | ChEBI | | 1-Arachidonoyl-gpi | HMDB | | 1-Arachidonoyl-glycero-3-phospho-(1'-myo-inositol) | HMDB | | 1-Arachidonoyl-glycero-3-phospho-(1’-myo-inositol) | HMDB | | 1-Arachidonoyl-lysophosphatidylinositol | HMDB | | GPI(20:4(5Z,8Z,11Z,14Z)) | HMDB | | GPI(20:4(5Z,8Z,11Z,14Z)/0:0) | HMDB | | GPI(20:4) | HMDB | | GPI(20:4n6) | HMDB | | GPI(20:4n6/0:0) | HMDB | | GPI(20:4W6) | HMDB | | GPI(20:4W6/0:0) | HMDB | | LPI(20:4(5Z,8Z,11Z,14Z)) | HMDB | | LPI(20:4(5Z,8Z,11Z,14Z)/0:0) | HMDB | | LPI(20:4) | HMDB | | LPI(20:4n6) | HMDB | | LPI(20:4n6/0:0) | HMDB | | LPI(20:4W6) | HMDB | | LPI(20:4W6/0:0) | HMDB | | LysoPI(20:4(5Z,8Z,11Z,14Z)) | HMDB | | LysoPI(20:4) | HMDB | | LysoPI(20:4n6) | HMDB | | LysoPI(20:4n6/0:0) | HMDB | | LysoPI(20:4W6) | HMDB | | LysoPI(20:4W6/0:0) | HMDB | | Lysophosphatidylinositol(20:4(5Z,8Z,11Z,14Z)) | HMDB | | Lysophosphatidylinositol(20:4(5Z,8Z,11Z,14Z)/0:0) | HMDB | | Lysophosphatidylinositol(20:4) | HMDB | | Lysophosphatidylinositol(20:4n6) | HMDB | | Lysophosphatidylinositol(20:4n6/0:0) | HMDB | | Lysophosphatidylinositol(20:4W6) | HMDB | | Lysophosphatidylinositol(20:4W6/0:0) | HMDB | | 1-Arachidonoyl-sn-glycero-3-phospho-D-myo-inositol | HMDB | | LysoPI(20:4(5Z,8Z,11Z,14Z)/0:0) | HMDB |
|
|---|
| Chemical Formula | C29H49O12P |
|---|
| Average Molecular Weight | 620.6659 |
|---|
| Monoisotopic Molecular Weight | 620.296163544 |
|---|
| IUPAC Name | [(2R)-2-hydroxy-3-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyloxy]propoxy]({[(1S,2R,3R,4S,5S,6R)-2,3,4,5,6-pentahydroxycyclohexyl]oxy})phosphinic acid |
|---|
| Traditional Name | (2R)-2-hydroxy-3-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyloxy]propoxy([(1S,2R,3R,4S,5S,6R)-2,3,4,5,6-pentahydroxycyclohexyl]oxy)phosphinic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OC[C@@H](O)COP(O)(=O)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C29H49O12P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(31)39-20-22(30)21-40-42(37,38)41-29-27(35)25(33)24(32)26(34)28(29)36/h6-7,9-10,12-13,15-16,22,24-30,32-36H,2-5,8,11,14,17-21H2,1H3,(H,37,38)/b7-6-,10-9-,13-12-,16-15-/t22-,24-,25-,26+,27-,28-,29-/m1/s1 |
|---|
| InChI Key | LXUGKKVCSTYZFK-HYNUQJCBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-acyl-sn-glycerol-3-phosphoinositols. These are glycerophosphoinositols where the glycerol is acylated only at position O-1 with a fatty acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoinositols |
|---|
| Direct Parent | 1-acyl-sn-glycerol-3-phosphoinositols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-acyl-sn-glycerol-3-phosphoinositol
- Inositol phosphate
- Dialkyl phosphate
- Cyclohexanol
- Fatty acid ester
- Cyclitol or derivatives
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Cyclic alcohol
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Alcohol
- Organooxygen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | - 1-acyl-sn-glycero-3-phospho-1D-myo-inositol (CHEBI:83053 )
|
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-114i-4394332000-963f8604b130c859e105 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00b9-6292026000-b4a12c27a9ecdfc43f6b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_16) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_17) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0odr-1479308000-0a9eff06b41d9339340b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-2295001000-1c172b906c5370c65d0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fs-7962000000-61fa6801db1783185bfe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uy0-0079116000-97490d18ac7ea4a85622 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zi9-5298001000-e7f90cc393e81a0969f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9021000000-f14f8fff63fd5515620f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000009000-8aab9b7bfb0c6c007542 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000009000-8aab9b7bfb0c6c007542 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-0339103000-3f30c6b537d6f4f010b3 | View in MoNA |
|---|
|
|---|