| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-05-06 19:44:08 UTC |
|---|
| Update Date | 2020-05-07 14:44:59 UTC |
|---|
| BMDB ID | BMDB0109728 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
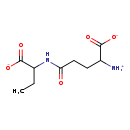
| Common Name | gamma-Glutamyl-2-aminobutyrate |
|---|
| Description | gamma-Glutamyl-2-aminobutyrate, also known as g-glu-abu(1-), belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. N-acyl-alpha amino acids and derivatives are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. Based on a literature review very few articles have been published on gamma-Glutamyl-2-aminobutyrate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-Glutamyl-2-aminobutyrate | Generator | | g-Glutamyl-2-aminobutyric acid | Generator | | gamma-Glutamyl-2-aminobutyric acid | Generator | | Γ-glutamyl-2-aminobutyrate | Generator | | Γ-glutamyl-2-aminobutyric acid | Generator | | g-Glu-abu(1-) | HMDB | | Γ-glu-abu(1-) | HMDB | | gamma-Glutamyl-2-aminobutyrate | ChEBI |
|
|---|
| Chemical Formula | C9H15N2O5 |
|---|
| Average Molecular Weight | 231.229 |
|---|
| Monoisotopic Molecular Weight | 231.098645171 |
|---|
| IUPAC Name | 2-azaniumyl-4-[(1-carboxypropyl)carbamoyl]butanoate |
|---|
| Traditional Name | 2-ammonio-4-[(1-carboxypropyl)carbamoyl]butanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC(NC(=O)CCC([NH3+])C([O-])=O)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C9H16N2O5/c1-2-6(9(15)16)11-7(12)4-3-5(10)8(13)14/h5-6H,2-4,10H2,1H3,(H,11,12)(H,13,14)(H,15,16)/p-1 |
|---|
| InChI Key | FUZOZPRKGAXGOB-UHFFFAOYSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. N-acyl-alpha amino acids and derivatives are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha amino acid or derivatives
- Alpha-amino acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Amino acid
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|