| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:28:57 UTC |
|---|
| Update Date | 2020-06-04 20:42:14 UTC |
|---|
| BMDB ID | BMDB0000343 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Hydroxyestrone |
|---|
| Description | 2-Hydroxyestrone belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Thus, 2-hydroxyestrone is considered to be a steroid lipid molecule. 2-Hydroxyestrone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 2-Hydroxyestrone participates in a number of enzymatic reactions, within cattle. In particular, 2-Hydroxyestrone can be biosynthesized from estrone; which is catalyzed by the enzyme cytochrome P450 1A1. In addition, S-Adenosylmethionine and 2-hydroxyestrone can be converted into 2-methoxyestrone and S-adenosylhomocysteine through its interaction with the enzyme catechol O-methyltransferase. In cattle, 2-hydroxyestrone is involved in the metabolic pathway called the estrone metabolism pathway. |
|---|
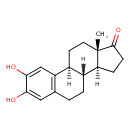
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dihydroxyestra-1,3,5(10)-trien-17-one | HMDB | | 2-OHE1 | HMDB | | Catecholestrone | HMDB | | 2-Hydroxyestrone, 4-(14)C-labeled | MeSH, HMDB |
|
|---|
| Chemical Formula | C18H22O3 |
|---|
| Average Molecular Weight | 286.3655 |
|---|
| Monoisotopic Molecular Weight | 286.15689457 |
|---|
| IUPAC Name | (1S,10R,11S,15S)-4,5-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| Traditional Name | (1S,10R,11S,15S)-4,5-dihydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| CAS Registry Number | 362-06-1 |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C(O)=C3 |
|---|
| InChI Identifier | InChI=1S/C18H22O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,19-20H,2-7H2,1H3/t11-,12+,14-,18-/m0/s1 |
|---|
| InChI Key | SWINWPBPEKHUOD-JPVZDGGYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- 2-hydroxysteroid
- Hydroxysteroid
- 17-oxosteroid
- Oxosteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ketone
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06vr-1980000000-fe43c8c5b3a4d07200c9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-016r-5449800000-4e55823f57ec07ec0913 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0w2i-0798000000-41a67b9322f4b2428372 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0f92-1920000000-1852b9bae72a1d8a9dc8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0zfs-2930000000-5911e48b958d80b00d92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-644377b19800b89d92e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0790000000-1a42c791a4695a613eac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-6690000000-645e51e9feba8eee90e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-42c0dd5f87119fd72c44 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-6509e54bf81b0caae551 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-1090000000-ff9544e7658c8bae039d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-c6a01b40f4853dcb1f7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07br-0590000000-21461e356ecdcb3c08c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0200-1910000000-030e5b518db4e2ad41fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-fcaf0435bbf678a207f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-3a4481ab24130f65a893 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kor-0190000000-13b24a7b3a6558370742 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|