| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:30:48 UTC |
|---|
| Update Date | 2020-05-11 20:50:29 UTC |
|---|
| BMDB ID | BMDB0000442 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (S)-3-Hydroxybutyric acid |
|---|
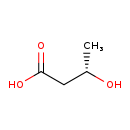
| Description | (S)-3-Hydroxybutyric acid, also known as (3S)-3-hydroxybutyrate or (S)-beta-hydroxybutanoic acid, belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. Based on a literature review a significant number of articles have been published on (S)-3-Hydroxybutyric acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-3-Hydroxybutyric acid | ChEBI | | (3S)-3-Hydroxybutyric acid | ChEBI | | (S)-3-Hydroxybutanoic acid | ChEBI | | (S)-3Hb | ChEBI | | (S)-beta-Hydroxybutyric acid | ChEBI | | L-(+)-3-Hydroxybutyric acid | ChEBI | | L-3-Hydroxybutyric acid | ChEBI | | (+)-3-Hydroxybutyrate | Generator | | (3S)-3-Hydroxybutyrate | Generator | | (S)-3-Hydroxybutanoate | Generator | | (S)-b-Hydroxybutyrate | Generator | | (S)-b-Hydroxybutyric acid | Generator | | (S)-beta-Hydroxybutyrate | Generator | | (S)-Β-hydroxybutyrate | Generator | | (S)-Β-hydroxybutyric acid | Generator | | L-(+)-3-Hydroxybutyrate | Generator | | L-3-Hydroxybutyrate | Generator | | (S)-3-Hydroxybutyrate | Generator | | (+)-3-Hydroxy-N-butyric acid | HMDB | | (3S)-3-Hydroxy-butanoate | HMDB | | (3S)-3-Hydroxy-butanoic acid | HMDB | | (S)-3-Hydroxy-2-methyl-propanoate | HMDB | | (S)-3-Hydroxy-2-methyl-propanoic acid | HMDB | | (S)-3-Hydroxy-butanoate | HMDB | | (S)-3-Hydroxy-butanoic acid | HMDB | | (S)-b-Hydroxyisobutyrate | HMDB | | (S)-b-Hydroxyisobutyric acid | HMDB | | (S)-beta-Hydroxyisobutyrate | HMDB | | (S)-beta-Hydroxyisobutyric acid | HMDB | | L-(+)-2-Methyl-hydracrylate | HMDB | | L-(+)-2-Methyl-hydracrylic acid | HMDB | | L-(+)-b-Hydroxyisobutyrate | HMDB | | L-(+)-b-Hydroxyisobutyric acid | HMDB | | L-(+)-beta-Hydroxyisobutyrate | HMDB | | L-(+)-beta-Hydroxyisobutyric acid | HMDB | | L-beta-Hydroxybutyrate | HMDB | | (3S)-3-Hydroxybutanoic acid | HMDB | | (S)-(+)-beta-Hydroxybutyric acid | HMDB | | (S)-(+)-Β-hydroxybutyric acid | HMDB | | (S)-beta-Hydroxybutanoic acid | HMDB | | (S)-Β-hydroxybutanoic acid | HMDB | | 3-Hydroxy-N-butyric acid | HMDB | | 3-Hydroxybutanoic acid | HMDB | | 3-Hydroxybutyric acid | HMDB | | L-beta-Hydroxybutyric acid | HMDB | | L-Β-hydroxybutyric acid | HMDB | | beta-Hydroxy-N-butyric acid | HMDB | | beta-Hydroxybutanoic acid | HMDB | | beta-Hydroxybutyric acid | HMDB | | Β-hydroxy-N-butyric acid | HMDB | | Β-hydroxybutanoic acid | HMDB | | Β-hydroxybutyric acid | HMDB | | (S)-3-Hydroxybutyric acid | HMDB |
|
|---|
| Chemical Formula | C4H8O3 |
|---|
| Average Molecular Weight | 104.1045 |
|---|
| Monoisotopic Molecular Weight | 104.047344122 |
|---|
| IUPAC Name | (3S)-3-hydroxybutanoic acid |
|---|
| Traditional Name | β-hydroxybutyrate,l |
|---|
| CAS Registry Number | 6168-83-8 |
|---|
| SMILES | C[C@H](O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m0/s1 |
|---|
| InChI Key | WHBMMWSBFZVSSR-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Short-chain hydroxy acid
- Beta-hydroxy acid
- Fatty acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 45 - 48 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0007-9000000000-5f169537ace358b06fd0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01ei-9710000000-2652bd46b41e50defdb0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-0a4i-9200000000-4156904e7472b5e97249 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-0a4i-9300000000-505ae46abb0c49c78b1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-0zfr-9600000000-dfed69c37c1a4d794440 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-9100000000-889e2968ad4fd52cdd92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-9000000000-6c660170b8f4aa6bcd02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-c39869c905b7e93b7f97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-9800000000-532ea53160d6ca2efcaa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pbi-9200000000-b2c59fd56b1a1d713ea9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-29e8d104108ac71cd640 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-de698d113a869245219a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-d964762034ff3766529f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-1c4d36fa24f00e6e9f6e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9200000000-61512767fe2a2778ee8c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-0006bf28af00ac9af39c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-58f5546067f0f7cf64bb | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|