| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:39:24 UTC |
|---|
| Update Date | 2020-05-21 16:28:50 UTC |
|---|
| BMDB ID | BMDB0000978 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
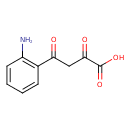
| Common Name | 4-(2-Aminophenyl)-2,4-dioxobutanoic acid |
|---|
| Description | 4-(2-Aminophenyl)-2,4-dioxobutanoic acid belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. 4-(2-Aminophenyl)-2,4-dioxobutanoic acid is possibly soluble (in water) and a strong basic compound (based on its pKa). 4-(2-Aminophenyl)-2,4-dioxobutanoic acid exists in all living species, ranging from bacteria to humans. 4-(2-Aminophenyl)-2,4-dioxobutanoic acid and L-glutamic acid can be biosynthesized from L-kynurenine and oxoglutaric acid; which is catalyzed by the enzyme kynurenine/alpha-aminoadipate aminotransferase, mitochondrial. In cattle, 4-(2-aminophenyl)-2,4-dioxobutanoic acid is involved in the metabolic pathway called the tryptophan metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(2-Aminophenyl)-2,4-dioxobutanoate | ChEBI | | 4-(2-Aminophenyl)-2,4-dioxo-butanoate | HMDB | | 4-(2-Aminophenyl)-2,4-dioxo-butanoic acid | HMDB | | 2-Amino-alpha,gamma-dioxobenzenebutanoic acid | HMDB | | 2-Amino-α,γ-dioxobenzenebutanoic acid | HMDB | | 4-(2-Aminophenyl)-2,4-dioxobutanoic acid | HMDB |
|
|---|
| Chemical Formula | C10H9NO4 |
|---|
| Average Molecular Weight | 207.1828 |
|---|
| Monoisotopic Molecular Weight | 207.053157781 |
|---|
| IUPAC Name | 4-(2-aminophenyl)-2,4-dioxobutanoic acid |
|---|
| Traditional Name | 4-(2-aminophenyl)-2,4-dioxobutanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | NC1=C(C=CC=C1)C(=O)CC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H9NO4/c11-7-4-2-1-3-6(7)8(12)5-9(13)10(14)15/h1-4H,5,11H2,(H,14,15) |
|---|
| InChI Key | CAOVWYZQMPNAFJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Butyrophenone
- Benzoyl
- Gamma-keto acid
- Aryl alkyl ketone
- Aniline or substituted anilines
- 1,3-diketone
- Monocyclic benzene moiety
- Alpha-keto acid
- Keto acid
- 1,3-dicarbonyl compound
- Benzenoid
- Alpha-hydroxy ketone
- Vinylogous amide
- Amino acid
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Primary amine
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02ml-9600000000-9ee4bc222cfbefcf7167 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-6940000000-0a58537f3d042b452b9a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0950000000-f6e0367966abff1dc873 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-a2c601ca3bb3e2f03be2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9500000000-7dd33609488e64596836 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1490000000-82e91d56bdce0e09cb73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-4930000000-4e754ee43c0a1b217738 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-ff4da0a8b66f352cf30d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-3920000000-ed3ee349719d21882219 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9200000000-5a28b87fef7bde9d37c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-5761d11f372a2a30748f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08mi-2930000000-680696b189e40364b3e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2900000000-e522d34ea0481d109d3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-ee62e52b5df8d4bab6ff | View in MoNA |
|---|
|
|---|