| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:39:30 UTC |
|---|
| Update Date | 2020-05-21 16:29:03 UTC |
|---|
| BMDB ID | BMDB0000988 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | S-Adenosylmethioninamine |
|---|
| Description | S-Adenosylmethioninamine, also known as decarboxy-adomet or dadomet, belongs to the class of organic compounds known as 5'-deoxy-5'-thionucleosides. These are 5'-deoxyribonucleosides in which the ribose is thio-substituted at the 5'position by a S-alkyl group. S-Adenosylmethioninamine exists in all living species, ranging from bacteria to plants to humans. Based on a literature review a small amount of articles have been published on S-Adenosylmethioninamine. |
|---|
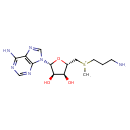
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulfonium | ChEBI | | (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulfonium cation | ChEBI | | (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulfonium salt | ChEBI | | [1-(Adenin-9-yl)-1,5-dideoxy-beta-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulfonium | ChEBI | | S-Adenosyl-(5')-3-methylthiopropylamine | ChEBI | | S-Adenosyl-3-methylthiopropylamine | ChEBI | | S-Adenosyl 3-(methylthio)propylamine | Kegg | | S-Adenosyl 3-(methylsulfanyl)propylamine | Kegg | | Decarboxy-adomet | Kegg | | (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulphonium | Generator | | (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulphonium cation | Generator | | (5-Deoxy-5-adenosyl)(3-aminopropyl)methylsulphonium salt | Generator | | [1-(Adenin-9-yl)-1,5-dideoxy-b-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulfonium | Generator | | [1-(Adenin-9-yl)-1,5-dideoxy-b-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulphonium | Generator | | [1-(Adenin-9-yl)-1,5-dideoxy-beta-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulphonium | Generator | | [1-(Adenin-9-yl)-1,5-dideoxy-β-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulfonium | Generator | | [1-(Adenin-9-yl)-1,5-dideoxy-β-D-ribofuranos-5-yl](3-aminopropyl)(methyl)sulphonium | Generator | | S-Adenosyl 3-(methylsulphanyl)propylamine | Generator | | S--Adenosylmethioninamine | HMDB | | (5-Deoxy-5-adenosyl)(3-aminopropyl) methylsulfonium salt | HMDB | | DAdoMet | HMDB | | Decarboxylated adomet | HMDB | | Decarboxylated S-adenosylmethionine | HMDB | | Decarboxylated sam | HMDB | | S-Adenosyl-L-methioninamine | HMDB | | S-5'-Deoxyadenosyl-(5')-3-methylthiopropylamine | HMDB | | S-Adenosyl(5')-3-methylthiopropylamine | HMDB | | (-)-S-Adenosyl-(5')-3-methylthiopropylamine | HMDB | | (-)-S-Adenosyl-(5’)-3-methylthiopropylamine | HMDB | | 5'-[(3-Aminopropyl)methylsulfonio]-5'-deoxyadenosine | HMDB | | 5’-[(3-aminopropyl)methylsulfonio]-5’-deoxyadenosine | HMDB | | S-Adenosyl-(5’)-3-methylthiopropylamine | HMDB | | S-Adenosyl-L-methionamine | HMDB | | S-Adenosylmethionamine | HMDB | | S-Methyl-S-adenosyl homocysteamine | HMDB | | S-Methyl-S-adenosylhomocysteamine | HMDB | | S-Methyladenosylhomocysteamine | HMDB | | S-Adenosylmethioninamine | HMDB |
|
|---|
| Chemical Formula | C14H23N6O3S |
|---|
| Average Molecular Weight | 355.436 |
|---|
| Monoisotopic Molecular Weight | 355.155234322 |
|---|
| IUPAC Name | {[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(3-aminopropyl)methylsulfanium |
|---|
| Traditional Name | decarboxylated sam |
|---|
| CAS Registry Number | 22365-13-5 |
|---|
| SMILES | C[S+](CCCN)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C14H23N6O3S/c1-24(4-2-3-15)5-8-10(21)11(22)14(23-8)20-7-19-9-12(16)17-6-18-13(9)20/h6-8,10-11,14,21-22H,2-5,15H2,1H3,(H2,16,17,18)/q+1/t8-,10-,11-,14-,24?/m1/s1 |
|---|
| InChI Key | ZUNBITIXDCPNSD-LSRJEVITSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 5'-deoxy-5'-thionucleosides. These are 5'-deoxyribonucleosides in which the ribose is thio-substituted at the 5'position by a S-alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | 5'-deoxyribonucleosides |
|---|
| Sub Class | 5'-deoxy-5'-thionucleosides |
|---|
| Direct Parent | 5'-deoxy-5'-thionucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 5'-deoxy-5'-thionucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Pentose monosaccharide
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- Imidolactam
- Pyrimidine
- Monosaccharide
- N-substituted imidazole
- Heteroaromatic compound
- Tetrahydrofuran
- Azole
- Imidazole
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Amine
- Alcohol
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06wi-7923000000-45e056b479c0e2cba364 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00ai-8291700000-8e1b09746b1c704e209f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0904000000-5a1b6a3a878c0630e6ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1900000000-70103a635ebc7c51a985 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3900000000-65b9306b33b0ffc5e9fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0916000000-8fc88153f214f58b3dc2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-8c8ee467ecf1a037a37b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mx-7900000000-b79675da812011642f0b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|