| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:39:49 UTC |
|---|
| Update Date | 2020-05-11 20:48:18 UTC |
|---|
| BMDB ID | BMDB0001008 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Biliverdin |
|---|
| Description | Biliverdin belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. Biliverdin is a very strong basic compound (based on its pKa). |
|---|
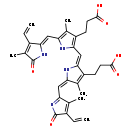
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Biliverdin IX | HMDB, MeSH | | 1,3,6,7-Tetramethyl-4,5-dicarboxyethyl-2,8-divinylbilenone | HMDB | | Biliverdin IX alpha | HMDB, MeSH | | Biliverdine | HMDB, MeSH | | Dehydrobilirubin | HMDB, MeSH | | Protobiliverdin IX | HMDB | | Uteroverdine | HMDB, MeSH | | IX, biliverdin | MeSH | | IX alpha, biliverdin | MeSH | | Ooecyan | MeSH | | alpha, Biliverdin IX | MeSH |
|
|---|
| Chemical Formula | C33H34N4O6 |
|---|
| Average Molecular Weight | 582.6463 |
|---|
| Monoisotopic Molecular Weight | 582.24783484 |
|---|
| IUPAC Name | 3-(2-{[(2Z,5E)-3-(2-carboxyethyl)-5-[(3-ethenyl-4-methyl-2-oxo-2H-pyrrol-5-yl)methylidene]-4-methyl-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| Traditional Name | 3-(2-{[(2Z,5E)-3-(2-carboxyethyl)-5-[(4-ethenyl-3-methyl-5-oxopyrrol-2-yl)methylidene]-4-methyl-1H-pyrrol-2-ylidene]methyl}-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| CAS Registry Number | 114-25-0 |
|---|
| SMILES | CC1=C(C=C)\C(NC1=O)=C\C1=C(C)C(CCC(O)=O)=C(N1)\C=C1/N\C(=C\C2=NC(=O)C(C=C)=C2C)C(C)=C1CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C33H34N4O6/c1-7-20-19(6)32(42)37-27(20)14-25-18(5)23(10-12-31(40)41)29(35-25)15-28-22(9-11-30(38)39)17(4)24(34-28)13-26-16(3)21(8-2)33(43)36-26/h7-8,13-15,34-35H,1-2,9-12H2,3-6H3,(H,37,42)(H,38,39)(H,40,41)/b24-13+,27-14-,28-15- |
|---|
| InChI Key | RCNSAJSGRJSBKK-YKSNQIBWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- Dipyrrin
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Pyrroline
- Heteroaromatic compound
- Carboxamide group
- Lactam
- N-acylimine
- Secondary carboxylic acid amide
- Azacycle
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Carboxylic acid
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
- Nucleus
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| Synthesis Reference | Mora, Maria E.; Bari, Sara E.; Awruch, Josefina; Delfino, Jose M. On how the conformation of biliverdins influences their reduction to bilirubins: A biological and molecular modeling study. Bioorganic & Medicinal Chemistry (2003), 11(21), 4661-467 |
|---|