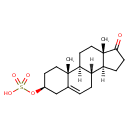
| (3-beta)-3-(Sulfooxy)androst-5-en-17-one | ChEBI |
| 17-Ketoandrost-5-en-3beta-yl sulfate | ChEBI |
| 17-Oxoandrost-5-en-3beta-yl hydrogen sulphate | ChEBI |
| 3-O-Sulfodehydroepiandrosterone | ChEBI |
| 3beta-Hydroxyandrost-5-en-17-one 3-sulfate | ChEBI |
| Androst-5-en-17-on-3beta-yl sulfuric acid | ChEBI |
| Dehydroepiandrosterone 3-sulfate | ChEBI |
| Dehydroepiandrosterone monosulfate | ChEBI |
| Dehydroepiandrosterone sulphate | ChEBI |
| Dehydroisoandrosterone sulfate | ChEBI |
| Dehydroisoandrosterone-3-sulfate | ChEBI |
| DHEA sulfate | ChEBI |
| DHEA-S | ChEBI |
| DHEAS | ChEBI |
| Prasterone sulfate | ChEBI |
| (3-b)-3-(Sulfooxy)androst-5-en-17-one | Generator |
| (3-b)-3-(Sulphooxy)androst-5-en-17-one | Generator |
| (3-beta)-3-(Sulphooxy)androst-5-en-17-one | Generator |
| (3-Β)-3-(sulfooxy)androst-5-en-17-one | Generator |
| (3-Β)-3-(sulphooxy)androst-5-en-17-one | Generator |
| 17-Ketoandrost-5-en-3b-yl sulfate | Generator |
| 17-Ketoandrost-5-en-3b-yl sulfuric acid | Generator |
| 17-Ketoandrost-5-en-3b-yl sulphate | Generator |
| 17-Ketoandrost-5-en-3b-yl sulphuric acid | Generator |
| 17-Ketoandrost-5-en-3beta-yl sulfuric acid | Generator |
| 17-Ketoandrost-5-en-3beta-yl sulphate | Generator |
| 17-Ketoandrost-5-en-3beta-yl sulphuric acid | Generator |
| 17-Ketoandrost-5-en-3β-yl sulfate | Generator |
| 17-Ketoandrost-5-en-3β-yl sulfuric acid | Generator |
| 17-Ketoandrost-5-en-3β-yl sulphate | Generator |
| 17-Ketoandrost-5-en-3β-yl sulphuric acid | Generator |
| 17-Oxoandrost-5-en-3b-yl hydrogen sulfate | Generator |
| 17-Oxoandrost-5-en-3b-yl hydrogen sulfuric acid | Generator |
| 17-Oxoandrost-5-en-3b-yl hydrogen sulphate | Generator |
| 17-Oxoandrost-5-en-3b-yl hydrogen sulphuric acid | Generator |
| 17-Oxoandrost-5-en-3beta-yl hydrogen sulfate | Generator |
| 17-Oxoandrost-5-en-3beta-yl hydrogen sulfuric acid | Generator |
| 17-Oxoandrost-5-en-3beta-yl hydrogen sulphuric acid | Generator |
| 17-Oxoandrost-5-en-3β-yl hydrogen sulfate | Generator |
| 17-Oxoandrost-5-en-3β-yl hydrogen sulfuric acid | Generator |
| 17-Oxoandrost-5-en-3β-yl hydrogen sulphate | Generator |
| 17-Oxoandrost-5-en-3β-yl hydrogen sulphuric acid | Generator |
| 3-O-Sulphodehydroepiandrosterone | Generator |
| 3b-Hydroxyandrost-5-en-17-one 3-sulfate | Generator |
| 3b-Hydroxyandrost-5-en-17-one 3-sulfuric acid | Generator |
| 3b-Hydroxyandrost-5-en-17-one 3-sulphate | Generator |
| 3b-Hydroxyandrost-5-en-17-one 3-sulphuric acid | Generator |
| 3beta-Hydroxyandrost-5-en-17-one 3-sulfuric acid | Generator |
| 3beta-Hydroxyandrost-5-en-17-one 3-sulphate | Generator |
| 3beta-Hydroxyandrost-5-en-17-one 3-sulphuric acid | Generator |
| 3Β-hydroxyandrost-5-en-17-one 3-sulfate | Generator |
| 3Β-hydroxyandrost-5-en-17-one 3-sulfuric acid | Generator |
| 3Β-hydroxyandrost-5-en-17-one 3-sulphate | Generator |
| 3Β-hydroxyandrost-5-en-17-one 3-sulphuric acid | Generator |
| Androst-5-en-17-on-3b-yl sulfate | Generator |
| Androst-5-en-17-on-3b-yl sulfuric acid | Generator |
| Androst-5-en-17-on-3b-yl sulphate | Generator |
| Androst-5-en-17-on-3b-yl sulphuric acid | Generator |
| Androst-5-en-17-on-3beta-yl sulfate | Generator |
| Androst-5-en-17-on-3beta-yl sulphate | Generator |
| Androst-5-en-17-on-3beta-yl sulphuric acid | Generator |
| Androst-5-en-17-on-3β-yl sulfate | Generator |
| Androst-5-en-17-on-3β-yl sulfuric acid | Generator |
| Androst-5-en-17-on-3β-yl sulphate | Generator |
| Androst-5-en-17-on-3β-yl sulphuric acid | Generator |
| Dehydroepiandrosterone 3-sulfuric acid | Generator |
| Dehydroepiandrosterone 3-sulphate | Generator |
| Dehydroepiandrosterone 3-sulphuric acid | Generator |
| Dehydroepiandrosterone monosulfuric acid | Generator |
| Dehydroepiandrosterone monosulphate | Generator |
| Dehydroepiandrosterone monosulphuric acid | Generator |
| Dehydroepiandrosterone sulfuric acid | Generator |
| Dehydroepiandrosterone sulphuric acid | Generator |
| Dehydroisoandrosterone sulfuric acid | Generator |
| Dehydroisoandrosterone sulphate | Generator |
| Dehydroisoandrosterone sulphuric acid | Generator |
| Dehydroisoandrosterone-3-sulfuric acid | Generator |
| Dehydroisoandrosterone-3-sulphate | Generator |
| Dehydroisoandrosterone-3-sulphuric acid | Generator |
| DHEA sulfuric acid | Generator |
| DHEA sulphate | Generator |
| DHEA sulphuric acid | Generator |
| Prasterone sulfuric acid | Generator |
| Prasterone sulphate | Generator |
| Prasterone sulphuric acid | Generator |
| (3beta)-3-(Sulfooxy)-androst-5-en-17-one | HMDB |
| 17-Oxoandrost-5-en-3-yl hydrogen sulfate | HMDB |
| 17-Oxoandrost-5-en-3-yl hydrogen sulphate | HMDB |
| 3-b-Hydroxyandrost-5-en-17-one 3-sulfate | HMDB |
| 3-b-Hydroxyandrost-5-en-17-one 3-sulphate | HMDB |
| 3-beta-Hydroxyandrost-5-en-17-one 3-sulfate | HMDB |
| 3-beta-Hydroxyandrost-5-en-17-one 3-sulphate | HMDB |
| 3b-Hydroxy-androst-5-en-17-one hydrogen sulfate | HMDB |
| 3b-Hydroxy-androst-5-en-17-one hydrogen sulphate | HMDB |
| 3beta-Hydroxy-androst-5-en-17-one hydrogen sulfate | HMDB |
| 3beta-Hydroxy-androst-5-en-17-one hydrogen sulphate | HMDB |
| Dehydroepiandrosterone-3-sulfate | HMDB |
| Dehydroepiandrosterone-3-sulphate | HMDB |
| Formylisoglutamic acid | HMDB |
| Prasterone-3-sulfate | HMDB |
| Prasterone-3-sulphate | HMDB |
| DHA sulfate | HMDB |
| Sulfate, dehydroisoandrosterone | HMDB |
| Sulfate, prasterone | HMDB |
| Sulfate, dhea | HMDB |
| Sulfate, dehydroepiandrosterone | HMDB |
| Sulfate, dha | HMDB |