| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:42:30 UTC |
|---|
| Update Date | 2020-05-21 16:28:49 UTC |
|---|
| BMDB ID | BMDB0001200 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N'-Formylkynurenine |
|---|
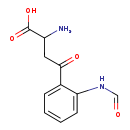
| Description | N'-n'-formylkynurenine belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. N'-n'-formylkynurenine is possibly soluble (in water) and a very strong basic compound (based on its pKa). N'-n'-formylkynurenine exists in all eukaryotes, ranging from yeast to humans. N'-n'-formylkynurenine participates in a number of enzymatic reactions, within cattle. In particular, N'-n'-formylkynurenine can be biosynthesized from L-tryptophan; which is mediated by the enzyme tryptophan 2,3-dioxygenase. In addition, N'-n'-formylkynurenine can be converted into formylanthranilic acid and L-alanine; which is catalyzed by the enzyme kynureninase. In cattle, n'-n'-formylkynurenine is involved in the metabolic pathway called the tryptophan metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(2-Formamidobenzoyl)alanine | ChEBI | | Formylkynurenine | ChEBI | | 3-(N-Formylanthraniloyl)-alanine | HMDB | | alpha-Amino-2-(formylamino)-gamma-oxo-benzenebutanoate | HMDB | | alpha-Amino-2-(formylamino)-gamma-oxo-benzenebutanoic acid | HMDB | | N'-formyl-kynurenine | HMDB | | N-Formyl-D-kynurenine | HMDB | | N-Formyl-delta-kynurenine | HMDB | | N-Formyl-L-kynurenine | HMDB | | N'-formylkynurenine, (R)-isomer | HMDB | | N'-formylkynurenine, (S)-isomer | HMDB | | N-Formylkynurenine | HMDB |
|
|---|
| Chemical Formula | C11H12N2O4 |
|---|
| Average Molecular Weight | 236.224 |
|---|
| Monoisotopic Molecular Weight | 236.079706882 |
|---|
| IUPAC Name | 2-amino-4-(2-formamidophenyl)-4-oxobutanoic acid |
|---|
| Traditional Name | N-formylkynurenine |
|---|
| CAS Registry Number | 1022-31-7 |
|---|
| SMILES | NC(CC(=O)C1=CC=CC=C1NC=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H12N2O4/c12-8(11(16)17)5-10(15)7-3-1-2-4-9(7)13-6-14/h1-4,6,8H,5,12H2,(H,13,14)(H,16,17) |
|---|
| InChI Key | BYHJHXPTQMMKCA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Butyrophenone
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Anilide
- Benzoyl
- Aryl alkyl ketone
- N-arylamide
- Gamma-keto acid
- Monocyclic benzene moiety
- Beta-aminoketone
- Benzenoid
- Keto acid
- Vinylogous amide
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Primary aliphatic amine
- Primary amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organopnictogen compound
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01vo-6910000000-4e6cdf4c6c61e2d98da1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0002-4920000000-af1cb05a967f8d904ba2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052o-0890000000-339c7859f1a6861150c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006w-2910000000-0f48ac25f1cb57097997 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-3900000000-4a9b4b58e68782a6f097 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-6290000000-33116552bdd41736319c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9450000000-a23cde743537668eb731 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9200000000-a4ce591059cb5d3f790c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0950000000-49699bc381d5dd6e3df1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-022d-0900000000-d42ed7b74bd169fc7cd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-6900000000-62029190bf6bbbd9f219 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-010c-0950000000-bf3ca7fe3436c6275730 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-5900000000-075fa36ebbd83bfc9d41 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-f99fb07e6bd62afd2894 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|