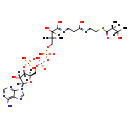
Showing metabocard for 2-Methyl-3-hydroxybutyryl-CoA (BMDB0001356)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 22:44:39 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-05-21 16:28:45 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0001356 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | 2-Methyl-3-hydroxybutyryl-CoA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 2-Methyl-3-hydroxybutyryl-CoA, also known as (S)-3-hydroxy-2-methylbutyryl-CoA, belongs to the class of organic compounds known as (s)-3-hydroxyacyl coas. These are organic compounds containing a (S)-3-hydroxyl acylated coenzyme A derivative. 2-Methyl-3-hydroxybutyryl-CoA is a strong basic compound (based on its pKa). 2-Methyl-3-hydroxybutyryl-CoA is a potentially toxic compound. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C26H44N7O18P3S | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 867.65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 867.167637865 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-3-({2-[(2-{[(2S,3S)-3-hydroxy-2-methylbutanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-({[hydroxy([hydroxy(3-hydroxy-3-({2-[(2-{[(2S,3S)-3-hydroxy-2-methylbutanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy)phosphoryl]oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxyphosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 52227-66-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | C[C@H](O)[C@H](C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C26H44N7O18P3S/c1-13(14(2)34)25(39)55-8-7-28-16(35)5-6-29-23(38)20(37)26(3,4)10-48-54(45,46)51-53(43,44)47-9-15-19(50-52(40,41)42)18(36)24(49-15)33-12-32-17-21(27)30-11-31-22(17)33/h11-15,18-20,24,34,36-37H,5-10H2,1-4H3,(H,28,35)(H,29,38)(H,43,44)(H,45,46)(H2,27,30,31)(H2,40,41,42)/t13-,14-,15+,18+,19+,20?,24+/m0/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | PEKYNTFSOBAABV-SYASONGASA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as (s)-3-hydroxyacyl coas. These are organic compounds containing a (S)-3-hydroxyl acylated coenzyme A derivative. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | (S)-3-hydroxyacyl CoAs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0001356 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB022575 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 389295 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | C04405 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | 43751 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | 6186 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 440326 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 15449 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Lipid transport and metabolism
- Specific function:
- Mitochondrial dehydrogenase that catalyzes the beta-oxidation at position 17 of androgens and estrogens and has 3-alpha-hydroxysteroid dehydrogenase activity with androsterone. Catalyzes the third step in the beta-oxidation of fatty acids. Carries out oxidative conversions of 7-alpha-OH and 7-beta-OH bile acids. Also exhibits 20-beta-OH and 21-OH dehydrogenase activities with C21 steroids. By interacting with intracellular amyloid-beta, it may contribute to the neuronal dysfunction associated with Alzheimer disease (AD). Essential for structural and functional integrity of mitochondria.
- Gene Name:
- HSD17B10
- Uniprot ID:
- O02691
- Molecular weight:
- 27140.0
Reactions
| 2-Methyl-3-hydroxybutyryl-CoA + NAD → 2-Methylacetoacetyl-CoA + NADH | details |
- General function:
- Not Available
- Specific function:
- Straight-chain enoyl-CoA thioesters from C4 up to at least C16 are processed, although with decreasing catalytic rate (By similarity). Has high substrate specificity for crotonyl-CoA and moderate specificity for acryloyl-CoA, 3-methylcrotonyl-CoA and methacrylyl-CoA. It is noteworthy that binds tiglyl-CoA, but hydrates only a small amount of this substrate (By similarity).
- Gene Name:
- ECHS1
- Uniprot ID:
- Q58DM8
- Molecular weight:
- 31243.0
Reactions
| Tiglyl-CoA + Water → 2-Methyl-3-hydroxybutyryl-CoA | details |