| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:44:49 UTC |
|---|
| Update Date | 2020-05-21 16:28:31 UTC |
|---|
| BMDB ID | BMDB0001368 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Mercaptopyruvic acid |
|---|
| Description | 3-3-3-mercaptopyruvic acid, also known as 3-mercaptopyruvic acid or 3-3-mercaptopyruvic acid, belongs to the class of organic compounds known as alpha-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the adjacent carbon. 3-3-3-mercaptopyruvic acid is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 3-3-3-mercaptopyruvic acid exists in all living organisms, ranging from bacteria to humans. 3-3-3-mercaptopyruvic acid participates in a number of enzymatic reactions, within cattle. In particular, 3-3-3-mercaptopyruvic acid and cyanide can be converted into pyruvic acid and thiocyanate through its interaction with the enzyme 3-3-mercaptopyruvic acid sulfurtransferase. In addition, 3-3-3-mercaptopyruvic acid can be biosynthesized from 3-mercaptolactic acid; which is catalyzed by the enzyme L-lactate dehydrogenase. In cattle, 3-3-mercaptopyruvic acid is involved in the metabolic pathway called the cysteine metabolism pathway. |
|---|
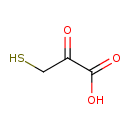
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Mercapto-2-oxopropanoic acid | ChEBI | | 3-Mercaptopyruvate | ChEBI | | Mercaptopyruvate | ChEBI | | 3-Mercapto-2-oxopropanoate | Generator | | Mercaptopyruvic acid | Generator | | 3-Mercapto-pyruvate | HMDB | | 3-Mercapto-pyruvic acid | HMDB | | beta-3-Mercapto-2-oxo-propanoate | HMDB | | beta-3-Mercapto-2-oxo-propanoic acid | HMDB | | beta-Mercaptopyruvate | HMDB | | beta-Mercaptopyruvic acid | HMDB | | beta-Thiopyruvate | HMDB | | beta-Thiopyruvic acid | HMDB | | Thiopyruvate | HMDB | | 3-Mercaptopyruvate monosodium salt | HMDB |
|
|---|
| Chemical Formula | C3H4O3S |
|---|
| Average Molecular Weight | 120.127 |
|---|
| Monoisotopic Molecular Weight | 119.988114684 |
|---|
| IUPAC Name | 2-oxo-3-sulfanylpropanoic acid |
|---|
| Traditional Name | β-mercaptopyruvic acid |
|---|
| CAS Registry Number | 2464-23-5 |
|---|
| SMILES | OC(=O)C(=O)CS |
|---|
| InChI Identifier | InChI=1S/C3H4O3S/c4-2(1-7)3(5)6/h7H,1H2,(H,5,6) |
|---|
| InChI Key | OJOLFAIGOXZBCI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the adjacent carbon. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Alpha-keto acids and derivatives |
|---|
| Direct Parent | Alpha-keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Alkylthiol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9000000000-b81f8808977132c3a8ac | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9300000000-5100fd5cbb3bf9df1e75 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-003r-9200000000-f1e0c53958ad51dc0068 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-001j-9000000000-60f6deb41f42078dd60c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-001i-9000000000-5dc69d3b59bd15290aef | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-9000000000-e1511333b005fa911312 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-00kf-9000000000-65854057c742d34c8dbb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-006w-9000000000-66939f30bca1061b4b83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-2900000000-0da521157f8235fe7758 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-7900000000-8c127be0565b54938cd2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-d4a8d07d451ba309b2e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-849e74ceb9308736ba6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-2900000000-5a713232367a68a966a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fk9-9300000000-564344466a63b943c6da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-9200000000-95772f7f626a811df489 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-32551e3b5faf0b1e7000 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-572c315b879edca344fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9400000000-8738d67598547a4b3429 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-4ba1ab7a6a1dc95bba23 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-6a9a04efc4b7bb2af1eb | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|