| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:46:41 UTC |
|---|
| Update Date | 2020-05-21 16:27:04 UTC |
|---|
| BMDB ID | BMDB0001490 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Vanylglycol |
|---|
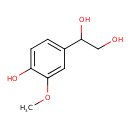
| Description | Vanylglycol, also known as MHPG or vanylglycol, belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. Vanylglycol is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Vanylglycol and pyrocatechol can be biosynthesized from 3,4-dihydroxyphenylglycol and guaiacol through its interaction with the enzyme catechol O-methyltransferase. In cattle, vanylglycol is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(4-Hydroxy-3-methoxyphenyl)-1,2-ethanediol | HMDB | | 3-Methoxy-4-hydroxyphenethylene glycol | HMDB | | 3-Methoxy-4-hydroxyphenyl glycol | HMDB | | 4-Hydroxy-3-methoxy-b-phenylglycol | HMDB | | 4-Hydroxy-3-methoxy-beta-phenylglycol | HMDB | | 4-Hydroxy-3-methoxyphenyl glycol | HMDB | | HMPG | HMDB | | Hydroxymethoxyphenylglycol | HMDB, MeSH | | Methoxyhydroxyphenylglycol | HMDB, MeSH | | MHPG | HMDB, MeSH | | MOPEG | HMDB, MeSH | | 4 Hydroxy 3 methoxyphenylethyleneglycol | MeSH, HMDB | | 4 Hydroxy 3 methoxyphenylglycol | MeSH, HMDB | | 4-Hydroxy-3-methoxyphenylethyleneglycol | MeSH, HMDB | | Methoxyhydroxyphenylglycol, (-)-isomer | MeSH, HMDB | | 4 Hydroxy 3 methoxyphenylethylene glycol | MeSH, HMDB | | 4-Hydroxy-3-methoxyphenylglycol | MeSH, HMDB | | Methoxyhydroxyphenylglycol, (+)-isomer | MeSH, HMDB | | 4-Hydroxy-3-methoxyphenylethylene glycol | MeSH, HMDB | | Methoxyhydroxyphenylglycol, (+-)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H12O4 |
|---|
| Average Molecular Weight | 184.1892 |
|---|
| Monoisotopic Molecular Weight | 184.073558872 |
|---|
| IUPAC Name | 1-(4-hydroxy-3-methoxyphenyl)ethane-1,2-diol |
|---|
| Traditional Name | methoxyhydroxyphenylglycol |
|---|
| CAS Registry Number | 534-82-7 |
|---|
| SMILES | COC1=CC(=CC=C1O)C(O)CO |
|---|
| InChI Identifier | InChI=1S/C9H12O4/c1-13-9-4-6(8(12)5-10)2-3-7(9)11/h2-4,8,10-12H,5H2,1H3 |
|---|
| InChI Key | FBWPWWWZWKPJFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Methoxyphenols |
|---|
| Direct Parent | Methoxyphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- 1,2-diol
- Ether
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2900000000-85ebe9a57a84ddca41b6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0079-7149000000-54b71f18882477571deb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0uxr-0900000000-fb2c82b5598c8c9bab3a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00di-0900000000-a449b185542d66dc0be8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-006x-9600000000-316d1194b9b0ed76d178 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-f104dd3883189a74b622 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0900000000-040295df7423a417edab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f72-3900000000-87310e73492350a05b5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-01cbe4fce361c48afabf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05ur-1900000000-b4ac6a118c11eb31a6ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-5900000000-4a1c057f8cdd445e7125 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004r-0900000000-df828b6c78ec969e404f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-4900000000-97f0c28e2ac7713a8fc5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-9100000000-ed2fd3623e179bd8e27f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-0900000000-9b55b059e2dc27b7380f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-2900000000-44ed0b094433b8f04721 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053u-6900000000-4de993e85915570061f3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|