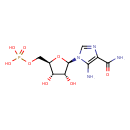| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:47:05 UTC |
|---|
| Update Date | 2020-05-21 16:27:08 UTC |
|---|
| BMDB ID | BMDB0001517 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | AICAR |
|---|
| Description | AICAR, also known as Z-nucleotide or ZMP, belongs to the class of organic compounds known as 1-ribosyl-imidazolecarboxamides. These are organic compounds containing the imidazole ring linked to a ribose ring through a 1-2 bond. AICAR is a strong basic compound (based on its pKa). AICAR exists in all living species, ranging from bacteria to humans. Within cattle, AICAR participates in a number of enzymatic reactions. In particular, fumaric acid and AICAR can be biosynthesized from SAICAR through the action of the enzyme adenylosuccinate lyase. In addition, 10-formyltetrahydrofolate and AICAR can be converted into tetrahydrofolic acid and phosphoribosyl formamidocarboxamide through its interaction with the enzyme bifunctional purine biosynthesis protein purh. In cattle, AICAR is involved in the metabolic pathway called the purine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(5'-Phosphoribosyl)-5-amino-4-imidazolecarboxamide | ChEBI | | 5'-Phospho-ribosyl-5-amino-4-imidazole carboxamide | ChEBI | | 5'-Phosphoribosyl-5-amino-4-imidazolecarboxamide | ChEBI | | 5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide | ChEBI | | 5-Aminoimidazole-4-carboxamide ribotide | ChEBI | | 5-Phosphoribosyl-4-carbamoyl-5-aminoimidazole | ChEBI | | Acadesine 5'-monophosphate | ChEBI | | AICA-Ribonucleotide | ChEBI | | 5-Amino-1-(5-phospho-beta-D-ribosyl)imidazole-4-carboxamide | Kegg | | Acadesine 5'-monophosphoric acid | Generator | | 5-Amino-1-(5-phospho-b-D-ribosyl)imidazole-4-carboxamide | Generator | | 5-Amino-1-(5-phospho-β-D-ribosyl)imidazole-4-carboxamide | Generator | | 5'-p-Ribosyl-5-amino-4-imidazole carboxamide | HMDB | | 5'-Phosphoribosyl-5-amino-4-imidazole carboxamide | HMDB | | 5-Amino-4-imidazolecarboxamide ribotide | HMDB | | AICA Ribonucleotide | HMDB | | Aminoimidazole carboxamide ribonucleotide | HMDB | | Z-Nucleotide | HMDB | | 4-Carboxy-5-aminoimidazole ribotide | HMDB | | 5-Amino-1-beta-D-ribofuranosylimidazole-4-carboxamide monophosphate | HMDB | | 5-Amino-4-imidazolecarboxamide ribofuranoside 5'-monophosphate | HMDB | | AICA Ribonucleotide, (D-ribofuranosyl)-isomer | HMDB | | AICAriboside 5'-monophosphate | HMDB | | CAIR | HMDB | | ZMP | HMDB | | (2R,3S,4R,5R)-5-(4-Carbam0yl-5-aminoimidazol-1-yl)-3,4-dihydroxyoxolan-2-yljmethyl dihydrogen phosphate | HMDB | | 5'-Phospho-beta-D-ribosyl-5-amino-4-imidazolecarboxamide | HMDB | | 5'-Phospho-β-D-ribosyl-5-amino-4-imidazolecarboxamide | HMDB | | 5-Amino-1-(5'-phosphofuranoribosyl)-4-imidazolecarboxamide | HMDB | | 5-Amino-1-(5-O-phosphono-beta-D-ribofuranosyl)-1H-imidazole-4-carboxamide | HMDB | | 5-Amino-1-(5-O-phosphono-β-D-ribofuranosyl)-1H-imidazole-4-carboxamide | HMDB | | 5-Amino-1-(5’-phosphofuranoribosyl)-4-imidazolecarboxamide | HMDB | | 5-Amino-1beta-D-ribofuranosylimidazole-4-carboxamide 5'-phosphate | HMDB | | 5-Amino-1β-D-ribofuranosylimidazole-4-carboxamide 5'-phosphate | HMDB | | 5-Amino-1β-D-ribofuranosylimidazole-4-carboxamide 5’-phosphate | HMDB | | 5-Amino-4-imidazolecarboxamide ribonucleoside 5'-monophosphate | HMDB | | 5-Amino-4-imidazolecarboxamide ribonucleoside 5’-monophosphate | HMDB | | 5-Amino-4-imidazolecarboxamide ribonucleotide | HMDB | | 5-Amino-4-imidazolecarboxamide riboside 5'-monophosphate | HMDB | | 5-Amino-4-imidazolecarboxamide riboside 5’-monophosphate | HMDB | | 5-Aminoimidazole-4-carboxamide ribonucleotide | HMDB | | 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-monophosphate | HMDB | | 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5’-monophosphate | HMDB | | 5’-Phospho-β-D-ribosyl-5-amino-4-imidazolecarboxamide | HMDB | | 5’-Phosphoribosyl-5-amino-4-imidazolecarboxamide | HMDB | | AICA Ribotide | HMDB | | AICAR | HMDB | | AICAR (5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-monophosphate) | HMDB | | AICAR (5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5'-monophosphate) | HMDB | | AICAR (5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5’-monophosphate) | HMDB | | Acadesine 5’-monophosphate | HMDB | | Aminoimidazolecarboxamide ribonucleotide | HMDB |
|
|---|
| Chemical Formula | C9H15N4O8P |
|---|
| Average Molecular Weight | 338.2112 |
|---|
| Monoisotopic Molecular Weight | 338.062749988 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | aica ribonucleotide |
|---|
| CAS Registry Number | 3031-94-5 |
|---|
| SMILES | NC(=O)C1=C(N)N(C=N1)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C9H15N4O8P/c10-7-4(8(11)16)12-2-13(7)9-6(15)5(14)3(21-9)1-20-22(17,18)19/h2-3,5-6,9,14-15H,1,10H2,(H2,11,16)(H2,17,18,19)/t3-,5-,6-,9-/m1/s1 |
|---|
| InChI Key | NOTGFIUVDGNKRI-UUOKFMHZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-ribosyl-imidazolecarboxamides. These are organic compounds containing the imidazole ring linked to a ribose ring through a 1-2 bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Imidazole ribonucleosides and ribonucleotides |
|---|
| Sub Class | 1-ribosyl-imidazolecarboxamides |
|---|
| Direct Parent | 1-ribosyl-imidazolecarboxamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-ribosyl-imidazolecarboxamide
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- 2-heteroaryl carboxamide
- Imidazole-4-carbonyl group
- Monoalkyl phosphate
- Aminoimidazole
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Tetrahydrofuran
- Primary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- 1,2-diol
- Carboxamide group
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Primary amine
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9613000000-e58f9c8b6a55e4f5a202 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0axr-6690400000-587ddf1606113df75d2d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-0009000000-7425edce1492dba01a40 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-000i-1019000000-4ca07d05181423b32ed1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004j-9300000000-5c28ca5957750fa338e3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9200000000-70e563a806a4c3a55e97 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9100000000-74be45171db0b5560214 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-004r-9104000000-230fd9fc88d41766b56e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-0119000000-9504e432c77e845f195d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-056r-1921000000-b59f42e588be20adfd16 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-2900000000-4725adb433c2ef4f7e01 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-4900000000-731f94a980a3ea830720 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03di-5900000000-e2c302b537e2a87761ca | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-2900000000-f0dad518d8c8a1fa40e2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-03di-1960000000-d34b556d9bc2997b36cc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-00c3-1596000000-85bb5fa83829ab8f259e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-02i7-1591000000-165d1c4d5b3bb7d6208e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-004i-3910000000-75666864aadfb515185f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-03di-3901000000-cf120ab9ca59f347fdd4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-03di-4900000000-249a325fdff8a321f364 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-004i-3910000000-9d01581a42f9d1ddbbaf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0914000000-84c17cba09b2fb11b4d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-1900000000-ebd6a0aeccf60882a9c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-7900000000-674e1cf6f4488a373690 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-9617000000-715abdd95d031de269f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9400000000-f3a4c70c454a0da6eb32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-3019a28f446b6ae4ee1f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|