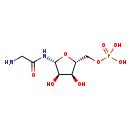| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:50:02 UTC |
|---|
| Update Date | 2020-05-19 22:01:26 UTC |
|---|
| BMDB ID | BMDB0002022 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Glycineamideribotide |
|---|
| Description | Glycineamideribotide, also known as GAR, belongs to the class of organic compounds known as glycinamide ribonucleotides. Glycinamide ribonucleotides are compounds in which the amide N atom of glycineamide is linked to the C-1 of a ribosyl (or deoxyribosyl) moiety. Nucleotides have a phosphate group linked to the C5 carbon of the ribose (or deoxyribose) moiety. Glycineamideribotide exists in all living species, ranging from bacteria to plants to humans. Based on a literature review very few articles have been published on Glycineamideribotide. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| GAR | Kegg | | N1-(5-Phospho-D-ribosyl)glycinamide | Kegg | | Glycinamide ribonucleotide | Kegg | | N1-(5-Phospho-beta-D-ribosyl)glycinamide | Kegg | | N1-(5-Phospho-b-D-ribosyl)glycinamide | Generator | | N1-(5-Phospho-β-D-ribosyl)glycinamide | Generator | | 5'-Phosphoribosyl-glycineamide | HMDB | | 5'-Phosphoribosylglycinamide | HMDB | | 5'-Phosphoribosylglycineamide | HMDB | | Glycineamide ribonucleotide | HMDB | | N(1)-(5-phospho-D-Ribosyl)glycinamide | HMDB | | N-Glycyl-5-O-phosphono-D-ribofuranosylamine | HMDB | | 2-Amino-N-(5-O-phosphono-beta-D-ribofuranosyl)acetamide | HMDB | | 2-Amino-N-(5-O-phosphono-β-D-ribofuranosyl)acetamide | HMDB | | 5-Phospho-beta-D-ribosyl-glycineamide | HMDB | | 5-Phospho-β-D-ribosyl-glycineamide | HMDB | | 5’-Phosphoribosylglycinamide | HMDB | | 5’-Phosphoribosylglycineamide | HMDB |
|
|---|
| Chemical Formula | C7H15N2O8P |
|---|
| Average Molecular Weight | 286.1764 |
|---|
| Monoisotopic Molecular Weight | 286.056601978 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(2-aminoacetamido)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | glycineamide ribonucleotide |
|---|
| CAS Registry Number | 10074-18-7 |
|---|
| SMILES | NCC(=O)N[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C7H15N2O8P/c8-1-4(10)9-7-6(12)5(11)3(17-7)2-16-18(13,14)15/h3,5-7,11-12H,1-2,8H2,(H,9,10)(H2,13,14,15)/t3-,5-,6-,7-/m1/s1 |
|---|
| InChI Key | OBQMLSFOUZUIOB-SHUUEZRQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycinamide ribonucleotides. Glycinamide ribonucleotides are compounds in which the amide N atom of glycineamide is linked to the C-1 of a ribosyl (or deoxyribosyl) moiety. Nucleotides have a phosphate group linked to the C5 carbon of the ribose (or deoxyribose) moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Glycinamide ribonucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Glycinamide ribonucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycinamide-ribonucleotide
- Pentose phosphate
- Pentose-5-phosphate
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Monosaccharide phosphate
- Pentose monosaccharide
- Monoalkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Tetrahydrofuran
- Secondary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- Carboxamide group
- 1,2-diol
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Alcohol
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Amine
- Organic oxygen compound
- Hydrocarbon derivative
- Primary amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000t-9420000000-d7fee78dd4657e2eba28 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00si-9541200000-4a23e3091baf72b01dc2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-053i-9460000000-f71dc4d8ec0f4da03819 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0560-9120000000-4770933b45387be6728c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9200000000-a6f2b9ab201fd0e69ee8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0170-9550000000-99520656892b8c415efb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-d6c860661b01956c80ac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-c2f2b85e6ec9caaa4408 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-2090000000-f6fcd99577c7e5d5d2ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9020000000-f5f361d4aef59382fc15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-962cd48ee45a1c95e446 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0980000000-6a9e014a86b77c5ad3c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-5920000000-cb0c5e3520ad275d3742 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-057j-9500000000-ab2ea8c5eb8a25044810 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|