| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:52:20 UTC |
|---|
| Update Date | 2020-05-21 16:28:40 UTC |
|---|
| BMDB ID | BMDB0002190 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5,6-Epoxy-8,11,14-eicosatrienoic acid |
|---|
| Description | 5,6-Epoxy-8,11,14-eicosatrienoic acid, also known as (+/-)5,6-epetre or 5,6-eet, belongs to the class of organic compounds known as epoxy fatty acids. These are fatty acids containing an oxirane ring as part of the aliphatic chain. Based on a literature review very few articles have been published on 5,6-Epoxy-8,11,14-eicosatrienoic acid. |
|---|
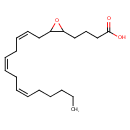
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+/-)5,6-epetre | ChEBI | | (8Z,11Z,14Z)-5,6-Epoxyeicosa-8,11,14-trienoic acid | ChEBI | | (8Z,11Z,14Z)-5,6-Epoxyicosa-8,11,14-trienoic acid | ChEBI | | 5,6-EpETrE | ChEBI | | 5,6-Epoxy-8,11,14-icosatrienoic acid | ChEBI | | 5,6-Epoxy-8Z,11Z,14Z-eicosatrienoic acid | ChEBI | | 5,6-Epoxy-8Z,11Z,14Z-icosatrienoic acid | ChEBI | | (8Z,11Z,14Z)-5,6-Epoxyeicosa-8,11,14-trienoate | Generator | | (8Z,11Z,14Z)-5,6-Epoxyicosa-8,11,14-trienoate | Generator | | 5,6-Epoxy-8,11,14-icosatrienoate | Generator | | 5,6-Epoxy-8Z,11Z,14Z-eicosatrienoate | Generator | | 5,6-Epoxy-8Z,11Z,14Z-icosatrienoate | Generator | | 5,6-Epoxy-8,11,14-eicosatrienoate | Generator | | 5,6-Eet | HMDB | | 5(6)-Oxido-8,11,14-eicosatrienoic acid | HMDB | | 5(6)-Oxidoeicosatrienoic acid | HMDB | | 5(6)Epoxyeicosatrienoic acid | HMDB | | 5,6-Epoxy-8,11,14-eicosatrienoic acid, (2alpha,3alpha(2Z,5Z,8Z))-isomer | HMDB |
|
|---|
| Chemical Formula | C20H32O3 |
|---|
| Average Molecular Weight | 320.4663 |
|---|
| Monoisotopic Molecular Weight | 320.23514489 |
|---|
| IUPAC Name | 4-{3-[(2Z,5Z,8Z)-tetradeca-2,5,8-trien-1-yl]oxiran-2-yl}butanoic acid |
|---|
| Traditional Name | 5(6)epoxyeicosatrienoic acid |
|---|
| CAS Registry Number | 81246-84-6 |
|---|
| SMILES | CCCCC\C=C/C\C=C/C\C=C/CC1OC1CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-15-18-19(23-18)16-14-17-20(21)22/h6-7,9-10,12-13,18-19H,2-5,8,11,14-17H2,1H3,(H,21,22)/b7-6-,10-9-,13-12- |
|---|
| InChI Key | VBQNSZQZRAGRIX-QNEBEIHSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as epoxy fatty acids. These are fatty acids containing an oxirane ring as part of the aliphatic chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Epoxy fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Epoxy fatty acid
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Ether
- Oxirane
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-9680000000-9277cd1ed0489c2f49f1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00bi-9343000000-8ebbc6b83f90c4773092 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1039000000-61ea0b5e6095e5a712d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-9452000000-e56fa17dede58805db86 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9420000000-4b7097444d03a2a2aa7a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0019000000-4e96728f18c66da2e4e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v00-3069000000-4536562ff67ce028e249 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9130000000-60d70cad2f9296348221 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-e9172f49e67d0b8f9e55 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-5119000000-d0ab8275ed285438b566 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9210000000-fa7840477fde0f1ed0fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-3329000000-2299a698c0d69c27f7f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ff0-9323000000-7bf675ec327ad703f03b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05qc-9000000000-a13d0bb6e0fb21746a1d | View in MoNA |
|---|
|
|---|