| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:53:27 UTC |
|---|
| Update Date | 2020-05-11 20:30:35 UTC |
|---|
| BMDB ID | BMDB0002274 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methylcobalamin |
|---|
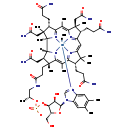
| Description | Methylcobalamin, also known as mecbl, belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. Methylcobalamin is formally rated as a possible carcinogen (by IARC 2B) and is also a potentially toxic compound. Based on a literature review a significant number of articles have been published on Methylcobalamin. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CO-METHYLCOBALAMIN | ChEBI | | MeCbl | ChEBI | | Mecobalamin | ChEBI | | Methylcob(III)alamin | ChEBI | | Methyl(III)cobalamin | HMDB |
|
|---|
| Chemical Formula | C63H91CoN13O14P |
|---|
| Average Molecular Weight | 1344.3823 |
|---|
| Monoisotopic Molecular Weight | 1343.587806391 |
|---|
| IUPAC Name | (10S,12R,13S,17R,23R,24R,25R,30S,35S,36S,40S,41S,42R,46R)-30,35,40-tris(2-carbamoylethyl)-24,36,41-tris(carbamoylmethyl)-46-hydroxy-12-(hydroxymethyl)-1,5,6,17,23,28,31,31,36,38,41,42-dodecamethyl-15,20-dioxo-11,14,16-trioxa-2lambda5,9,19,26,43lambda5,44lambda5,45lambda5-heptaaza-15lambda5-phospha-1-cobaltadodecacyclo[27.14.1.1^{1,34}.1^{2,9}.1^{10,13}.0^{1,26}.0^{3,8}.0^{23,27}.0^{25,42}.0^{32,44}.0^{39,43}.0^{37,45}]heptatetraconta-2(47),3,5,7,27,29(44),32,34(45),37,39(43)-decaene-2,43,44,45-tetrakis(ylium)-1,1,1-triuid-15-olate |
|---|
| Traditional Name | (10S,12R,13S,17R,23R,24R,25R,30S,35S,36S,40S,41S,42R,46R)-30,35,40-tris(2-carbamoylethyl)-24,36,41-tris(carbamoylmethyl)-46-hydroxy-12-(hydroxymethyl)-1,5,6,17,23,28,31,31,36,38,41,42-dodecamethyl-15,20-dioxo-11,14,16-trioxa-2lambda5,9,19,26,43lambda5,44lambda5,45lambda5-heptaaza-15lambda5-phospha-1-cobaltadodecacyclo[27.14.1.1^{1,34}.1^{2,9}.1^{10,13}.0^{1,26}.0^{3,8}.0^{23,27}.0^{25,42}.0^{32,44}.0^{39,43}.0^{37,45}]heptatetraconta-2(47),3,5,7,27,29(44),32,34(45),37,39(43)-decaene-2,43,44,45-tetrakis(ylium)-1,1,1-triuid-15-olate |
|---|
| CAS Registry Number | 13422-55-4 |
|---|
| SMILES | C1(CC[C@@]2([C@@H](CC(N)=O)[C@@]3([C@@]4([N+]5=C([C@H]([C@@]4(CC(N)=O)C)CCC(N)=O)C(C)=C4[N+]6=C(C=C7[N+]8=C([C@H](C7(C)C)CCC(N)=O)C(C)=C2N3[Co-3]568([N+]2=CN([C@H]3O[C@@H]([C@@H](OP(O[C@@H](CN1)C)([O-])=O)[C@H]3O)CO)C1=CC(C)=C(C=C21)C)C)[C@H]([C@@]4(CC(N)=O)C)CCC(N)=O)C)[H])C)=O |
|---|
| InChI Identifier | InChI=1S/C62H90N13O14P.CH3.Co/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H15,63,64,65,66,67,68,69,71,72,73,74,77,78,79,80,81,82,83,85,86);1H3;/q;;+2/p-2/t31-,34-,35-,36-,37+,41-,52-,53-,56-,57+,59-,60+,61+,62+;;/m1../s1 |
|---|
| InChI Key | JEWJRMKHSMTXPP-WZHZPDAFSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Cobalamin derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cobalamin
- Metallotetrapyrrole skeleton
- Pentose phosphate
- Monosaccharide phosphate
- Benzimidazole
- Benzenoid
- Fatty amide
- Organic phosphoric acid derivative
- N-substituted imidazole
- Fatty acyl
- Monosaccharide
- Pyrrolidine
- Pyrroline
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Carboxamide group
- Secondary carboxylic acid amide
- Primary carboxylic acid amide
- Lactam
- Carboxylic acid derivative
- Oxacycle
- Metalloheterocycle
- Azacycle
- Organic metal salt
- Organic transition metal salt
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organic salt
- Alcohol
- Organic transition metal moeity
- Organometallic compound
- Organonitrogen compound
- Organooxygen compound
- Transition metal alkyl
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0006-0009000000-270ebe7bf52ebc393bc3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0006-0009000000-837bcde718484550d5d7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-0009000000-9cdd3a31b906175760ad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f95-0089000000-105b8165c01a315a1c06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ke9-0049000000-874b2f636228deaa8738 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-0090000000-8fa0175ad99d0f3bcd2f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-0089000000-010c91e5b4f5bcb69026 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adj-1094000000-c827499f8629edca78e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-7092000000-cfd079b185ddc58fcf85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-aa82df0e0d556ebbbf0a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1094000000-bcae5ec9260f44330c24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0096000000-cdac342b4c786996dd29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-4b9d5321bce24e41c6b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-0098000000-588795fd3890fc7202c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-6079000000-76a3506aaf1ee44d2d67 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|