| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:08 UTC |
|---|
| Update Date | 2020-04-22 15:11:32 UTC |
|---|
| BMDB ID | BMDB0002916 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Nitrocatechol |
|---|
| Description | 4-Nitrocatechol belongs to the class of organic compounds known as nitrophenols. Nitrophenols are compounds containing a nitrophenol moiety, which consists of a benzene ring bearing both a hydroxyl group and a nitro group on two different ring carbon atoms. 4-Nitrocatechol exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on 4-Nitrocatechol. |
|---|
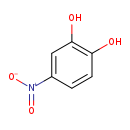
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dihydroxy-4-nitrobenzene | ChEBI | | 4-Nitropyrocatechol | ChEBI | | 3,4-Dihydroxy-1-nitrobenzene | HMDB | | 3,4-Dihydroxynitrobenzene | HMDB | | 4-nitro-1,2-Benzenediol | HMDB | | 4-nitro-1,2-Dihydroxybenzene | HMDB | | 4-nitro-Pyrocatechol4-nitropyrocatechol NSC 80651 | HMDB | | 4-Nitrobenzcatechin | HMDB | | P-Nitrocatechol | HMDB | | 2-Hydroxy-4-nitrophenolate | HMDB | | 4-Nitro-1,2-benzenediol | HMDB | | 4-Nitro-1,2-dihydroxybenzene | HMDB | | 4-Nitrocatechol | HMDB | | p-Nitrocatechol | HMDB |
|
|---|
| Chemical Formula | C6H5NO4 |
|---|
| Average Molecular Weight | 155.1082 |
|---|
| Monoisotopic Molecular Weight | 155.021857653 |
|---|
| IUPAC Name | 4-nitrobenzene-1,2-diol |
|---|
| Traditional Name | 4-nitrocatechol |
|---|
| CAS Registry Number | 3316-09-4 |
|---|
| SMILES | OC1=C(O)C=C(C=C1)[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/C6H5NO4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H |
|---|
| InChI Key | XJNPNXSISMKQEX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitrophenols. Nitrophenols are compounds containing a nitrophenol moiety, which consists of a benzene ring bearing both a hydroxyl group and a nitro group on two different ring carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Nitrophenols |
|---|
| Direct Parent | Nitrophenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nitrophenol
- Nitrobenzene
- Nitroaromatic compound
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- C-nitro compound
- Organic nitro compound
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 174 - 176 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.66 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001s-1950000000-faaf15fd1ec113f2b54e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001s-1950000000-faaf15fd1ec113f2b54e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001s-1950000000-faaf15fd1ec113f2b54e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001s-1950000000-faaf15fd1ec113f2b54e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4900000000-02b39e2d157c3829375f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-8390000000-9ce3af1465a211c62c08 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0fk9-1900000000-20992826e1c86b042245 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-cf479fcf220611c7c282 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-a438cfc44009c80ae2b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f92-3900000000-6a688f91418ed25d4aad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-3a1438af5cfdd1a76453 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-64163bd65bd38adfb2cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-3900000000-f9fb9e7b1ada6519c087 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-dd65e28b5507a1abfb0e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2900000000-ff3f84d5fc5d45d608e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9000000000-db86a62064c6c13c1639 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-c23d0f4048cedb728ea2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2900000000-0a7ee2badf00f8f5f00e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9000000000-a0441e6336543cb88423 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|