| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:06:06 UTC |
|---|
| Update Date | 2020-05-21 16:28:47 UTC |
|---|
| BMDB ID | BMDB0003791 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
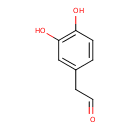
| Common Name | 3,4-Dihydroxyphenylacetaldehyde |
|---|
| Description | 3,4-Dihydroxyphenylacetaldehyde, also known as 3,4-dihydroxyphenylacetaldehyde or 3,4-dihydroxyphenylacetaldehyde, belongs to the class of organic compounds known as phenylacetaldehydes. Phenylacetaldehydes are compounds containing a phenylacetaldehyde moiety, which consists of a phenyl group substituted at the second position by an acetalydehyde. 3,4-Dihydroxyphenylacetaldehyde is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 3,4-Dihydroxyphenylacetaldehyde exists in all living organisms, ranging from bacteria to humans. 3,4-Dihydroxyphenylacetaldehyde participates in a number of enzymatic reactions, within cattle. In particular, 3,4-Dihydroxyphenylacetaldehyde can be biosynthesized from dopamine; which is catalyzed by the enzyme amine oxidase [flavin-containing] a. In addition, 3,4-Dihydroxyphenylacetaldehyde can be converted into 3,4-dihydroxybenzeneacetic acid; which is mediated by the enzyme 3,4-dihydroxyphenylacetaldehyde. In cattle, 3,4-dihydroxyphenylacetaldehyde is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dihydroxyphenyl)ethanal | ChEBI | | DOPAL | ChEBI | | Protocatechuatealdehyde | ChEBI | | DHPAA aldehyde | MeSH | | 3,4-Dihydroxy-benzeneacetaldehyde | HMDB |
|
|---|
| Chemical Formula | C8H8O3 |
|---|
| Average Molecular Weight | 152.1473 |
|---|
| Monoisotopic Molecular Weight | 152.047344122 |
|---|
| IUPAC Name | 2-(3,4-dihydroxyphenyl)acetaldehyde |
|---|
| Traditional Name | dopal |
|---|
| CAS Registry Number | 5707-55-1 |
|---|
| SMILES | OC1=C(O)C=C(CC=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H8O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,4-5,10-11H,3H2 |
|---|
| InChI Key | IADQVXRMSNIUEL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylacetaldehydes. Phenylacetaldehydes are compounds containing a phenylacetaldehyde moiety, which consists of a phenyl group substituted at the second position by an acetalydehyde. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylacetaldehydes |
|---|
| Direct Parent | Phenylacetaldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylacetaldehyde
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Alpha-hydrogen aldehyde
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.005 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-3900000000-7f82d1d31cd24be9e865 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00e9-8390000000-bba6c4376314951fb5ee | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-19827d81a06a62c2b70a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-2900000000-479d55c57638faa6d9cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9400000000-cadc5e95515b4cecf1f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-b0ef024b4e5895ddea08 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2900000000-352f5ee2c302c824867e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-96da2068f1ed3e30ae47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pb9-0900000000-588f3b3d236131a4fa7a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3900000000-d3765e83b72ff4e9c933 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-9100000000-63064bac00f3091d89c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-b75a7231958cf1fc53ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-ed865ca228b5007b7ed4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9100000000-7cc4c0962b7233330b8c | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|