| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:07:06 UTC |
|---|
| Update Date | 2020-05-21 16:29:04 UTC |
|---|
| BMDB ID | BMDB0003959 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 19-Oxotestosterone |
|---|
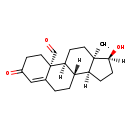
| Description | 19-Oxotestosterone belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. 19-Oxotestosterone exists as a solid, possibly soluble (in water), and an extremely weak basic (essentially neutral) compound (based on its pKa) molecule. 19-Oxotestosterone participates in a number of enzymatic reactions, within cattle. In particular, 19-Oxotestosterone can be converted into estradiol and formic acid through its interaction with the enzyme cytochrome P450 19A1. In addition, 19-Oxotestosterone can be biosynthesized from 19-hydroxytestosterone; which is catalyzed by the enzyme cytochrome P450 19A1. In cattle, 19-oxotestosterone is involved in the metabolic pathway called the androgen and estrogen metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C19H26O3 |
|---|
| Average Molecular Weight | 302.4079 |
|---|
| Monoisotopic Molecular Weight | 302.188194698 |
|---|
| IUPAC Name | (1S,2S,10S,11S,14S,15S)-14-hydroxy-15-methyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-ene-2-carbaldehyde |
|---|
| Traditional Name | 19-oxotestosterone |
|---|
| CAS Registry Number | 4075-13-2 |
|---|
| SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C=O |
|---|
| InChI Identifier | InChI=1S/C19H26O3/c1-18-8-7-16-14(15(18)4-5-17(18)22)3-2-12-10-13(21)6-9-19(12,16)11-20/h10-11,14-17,22H,2-9H2,1H3/t14-,15-,16-,17-,18-,19+/m0/s1 |
|---|
| InChI Key | TXNCGXATKOMEQC-KOUJMVCDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 19-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic alcohol
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aldehyde
- Organic oxide
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
- Myelin sheath
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-1190000000-38a46782f3a05e410638 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0592-2169000000-be11c2989577a025bceb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0095000000-f1b4c5073a1a349142cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ktr-0191000000-787c331104e02697f82e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-4290000000-c3ebd3f84fc5f2a0f1ad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0039000000-fcbe7a10b70f243584ca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-0098000000-88bd42d7a3c0ee3d7238 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-0090000000-e5ab8ef14971dd3b053a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-ab4e4333433d95463751 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0190000000-58b8fdd5c28b55834da9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aba-0900000000-a07aa8a9e25f2da78867 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-e1aa2d5a1d9cdeff04e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pi0-0093000000-1d8018c21ff4c38691c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0290000000-794f96d5ceb67c29f858 | View in MoNA |
|---|
|
|---|