| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:07:32 UTC |
|---|
| Update Date | 2020-05-21 16:28:47 UTC |
|---|
| BMDB ID | BMDB0004061 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Methoxy-4-hydroxyphenylglycolaldehyde |
|---|
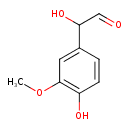
| Description | 3-Methoxy-4-hydroxyphenylglycolaldehyde belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. 3-Methoxy-4-hydroxyphenylglycolaldehyde is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 3-Methoxy-4-hydroxyphenylglycolaldehyde exists in all living organisms, ranging from bacteria to humans. 3-Methoxy-4-hydroxyphenylglycolaldehyde participates in a number of enzymatic reactions, within cattle. In particular, 3-Methoxy-4-hydroxyphenylglycolaldehyde can be biosynthesized from normetanephrine through its interaction with the enzyme amine oxidase [flavin-containing] a. In addition, 3-Methoxy-4-hydroxyphenylglycolaldehyde can be biosynthesized from metanephrine; which is mediated by the enzyme amine oxidase [flavin-containing] a. In cattle, 3-methoxy-4-hydroxyphenylglycolaldehyde is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-3-methoxy mandelaldehyde | HMDB | | 4-Hydroxy-3-methoxymandelaldehyde | HMDB | | 4-Hydroxy-3-methoxyphenylglycolaldehyde | HMDB | | a,4-Dihydroxy-3-methoxy-benzeneacetaldehyde | HMDB |
|
|---|
| Chemical Formula | C9H10O4 |
|---|
| Average Molecular Weight | 182.1733 |
|---|
| Monoisotopic Molecular Weight | 182.057908808 |
|---|
| IUPAC Name | 2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)acetaldehyde |
|---|
| Traditional Name | 2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)acetaldehyde |
|---|
| CAS Registry Number | 17592-23-3 |
|---|
| SMILES | COC1=C(O)C=CC(=C1)C(O)C=O |
|---|
| InChI Identifier | InChI=1S/C9H10O4/c1-13-9-4-6(8(12)5-10)2-3-7(9)11/h2-5,8,11-12H,1H3 |
|---|
| InChI Key | VISAJVAPYPFKCL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Methoxyphenols |
|---|
| Direct Parent | Methoxyphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methoxyphenol
- Phenylacetaldehyde
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Monocyclic benzene moiety
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Ether
- Aromatic alcohol
- Organooxygen compound
- Aldehyde
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 0.517 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2900000000-c3b751a80e98556beecd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-5193000000-6e22ae7f509439b91a97 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-241e0f5c9b5867f24f80 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fsi-0900000000-ad05d32557d49f4e6853 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abj-6900000000-3b0305c2b5932c3966b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-6784d8af6231fa270603 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kn9-0900000000-8395d2966f8d730d3dd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7900000000-348d28ad54efde88e76b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-e0b0d2106fad76afa1fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pi3-3900000000-cdf305b3288279dea0a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-5900000000-4c8fbae5388a3e0a5100 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0900000000-656cb77ee0208456f90b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-4900000000-e5c44a99eda118e291d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fj3-9500000000-b4df271139281b8e52ff | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|