| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:08:26 UTC |
|---|
| Update Date | 2020-05-21 16:28:55 UTC |
|---|
| BMDB ID | BMDB0004193 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N1-Methyl-2-pyridone-5-carboxamide |
|---|
| Description | N1-Methyl-2-pyridone-5-carboxamide, also known as N1-methyl-2-pyridone-5-carboxamide, belongs to the class of organic compounds known as nicotinamides. These are heterocyclic aromatic compounds containing a pyridine ring substituted at position 3 by a carboxamide group. N1-Methyl-2-pyridone-5-carboxamide is possibly soluble (in water) and a moderately basic compound (based on its pKa). N1-Methyl-2-pyridone-5-carboxamide exists in all living organisms, ranging from bacteria to humans. N1-Methyl-2-pyridone-5-carboxamide can be biosynthesized from 1-methylnicotinamide; which is catalyzed by the enzyme aldehyde oxidase. In cattle, N1-methyl-2-pyridone-5-carboxamide is involved in the metabolic pathway called the nicotinate and nicotinamide metabolism pathway. N1-Methyl-2-pyridone-5-carboxamide is a potentially toxic compound. |
|---|
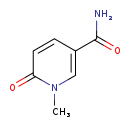
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methyl-5-carboxylamide-2-pyridone | ChEBI | | N'-methyl-2-pyridone-5-carboxamide | ChEBI | | N(1)-Methyl-2-pyridone-5-carboxamide | ChEBI | | N-Methyl-2-pyridone-5-carboxamide | ChEBI | | N'Methyl-2-pyridone-5-carboxamide | HMDB |
|
|---|
| Chemical Formula | C7H8N2O2 |
|---|
| Average Molecular Weight | 152.1506 |
|---|
| Monoisotopic Molecular Weight | 152.05857751 |
|---|
| IUPAC Name | 1-methyl-6-oxo-1,6-dihydropyridine-3-carboxamide |
|---|
| Traditional Name | 1-methyl-6-oxopyridine-3-carboxamide |
|---|
| CAS Registry Number | 701-44-0 |
|---|
| SMILES | CN1C=C(C=CC1=O)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C7H8N2O2/c1-9-4-5(7(8)11)2-3-6(9)10/h2-4H,1H3,(H2,8,11) |
|---|
| InChI Key | JLQSXXWTCJPCBC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nicotinamides. These are heterocyclic aromatic compounds containing a pyridine ring substituted at position 3 by a carboxamide group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Nicotinamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyridinecarboxylic acid derivative
- Nicotinamide
- Dihydropyridine
- Pyridinone
- Hydropyridine
- Heteroaromatic compound
- Lactam
- Carboximidic acid
- Carboximidic acid derivative
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zg0-4900000000-83295d8ad70d3d55646e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0gdi-9000000000-6078413def74bf56f076 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-3900000000-36bf456e144d30bcfd8c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0n4i-9300000000-af2a0597ef17437f3f98 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-fb97e1ad0bf72b8f81ef | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-0udi-0900000000-3df31b2e00bc83a4103e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0a4j-9500000000-843d3cb23381be7a74be | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-e43af86f5bf6d3983d06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-b64dc5db3a8f82da3c8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ik9-1900000000-7da53351adedc1e67e49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zir-9300000000-28053d0ec9ded55ae3cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1900000000-e93df4f3c2c8f4de0322 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-2900000000-36e016881280d17eaae6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-282653b830a8366d9872 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-a9caea9efa55584355b6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0900000000-d5151b931abbde60cc77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-6e22a1ee0ef6d5f765b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0900000000-3eb746bbbe14af3a4899 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pbi-1900000000-7d7d3dada7dd4c01c714 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9000000000-03f374d0eab3d5245833 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|