| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:08:51 UTC |
|---|
| Update Date | 2020-05-11 20:49:31 UTC |
|---|
| BMDB ID | BMDB0004246 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Bradykinin |
|---|
| Description | Bradykinin, also known as BK or RPPGFSPFR, belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Bradykinin is a very strong basic compound (based on its pKa). Bradykinin exists in all living organisms, ranging from bacteria to humans. |
|---|
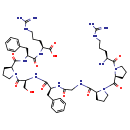
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Arg-pro-pro-gly-phe-ser-pro-phe-arg | ChEBI | | BK | ChEBI | | L-Arg-L-pro-L-pro-gly-L-phe-L-ser-L-pro-L-phe-L-arg | ChEBI | | L-Bradykinin | ChEBI | | RPPGFSPFR | ChEBI | | Callidin I | HMDB | | Kallidin 9 | HMDB | | Kallidin I | HMDB | | L-Arginyl-L-prolyl-L-prolylglycyl-L-phenylalanyl-L-seryl-L-prolyl-L-phenylalanyl-L-arginine | HMDB | | Bradykinin acetate, (9-D-arg)-isomer | HMDB | | Bradykinin hydrochloride | HMDB | | Bradykinin triacetate | HMDB | | Bradykinin, (2-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (8-D-phe)-isomer | HMDB | | Arg pro pro gly phe ser pro phe arg | HMDB | | Bradykinin, (1-D-arg)-isomer | HMDB | | Bradykinin, (2-D-pro-3-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (3-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (5-D-phe)-isomer | HMDB | | Bradykinin, (5-D-phe-8-D-phe)-isomer | HMDB | | Bradykinin, (6-D-ser)-isomer | HMDB | | Bradykinin, (7-D-pro)-isomer | HMDB | | Bradykinin, (9-D-arg)-isomer | HMDB | | Bradykinin diacetate | HMDB | | Bradykinin, (2-D-pro)-isomer | HMDB | | Bradykinin, (3-D-pro)-isomer | HMDB |
|
|---|
| Chemical Formula | C50H73N15O11 |
|---|
| Average Molecular Weight | 1060.2085 |
|---|
| Monoisotopic Molecular Weight | 1059.561398253 |
|---|
| IUPAC Name | (2S)-2-[(2S)-2-{[(2S)-1-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-1-[(2S)-2-amino-5-carbamimidamidopentanoyl]pyrrolidine-2-carbonyl]pyrrolidin-2-yl]formamido}acetamido)-3-phenylpropanamido]-3-hydroxypropanoyl]pyrrolidin-2-yl]formamido}-3-phenylpropanamido]-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | L-bradykinin |
|---|
| CAS Registry Number | 58-82-2 |
|---|
| SMILES | N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C50H73N15O11/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 |
|---|
| InChI Key | QXZGBUJJYSLZLT-FDISYFBBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Phenylalanine or derivatives
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Fatty amide
- Tertiary carboxylic acid amide
- Pyrrolidine
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Guanidine
- Amino acid
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboximidamide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Alcohol
- Primary aliphatic amine
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9605021310-963d37a5c0a6a8c3b581 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-4914021100-da28e55acbc5e55e7615 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9824010200-b5b2c55602c29375ed1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ard-9000000008-70e85b5126294f4866f8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4m-8001001009-d8431ee82b28629bac33 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9001111000-9de36ae5c0196bc1ca98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-eb21ccd98d434e431a1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-9000210241-b63ee10d53ba3efc65cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022l-9780340000-45c697c4a631655e2811 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-6f7d24ea48539f7cd9fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-9000100016-0338af4c69622fd514d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-9003871073-646bd5ef5605a552ce67 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|