| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:10:01 UTC |
|---|
| Update Date | 2020-04-22 15:13:58 UTC |
|---|
| BMDB ID | BMDB0004662 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | S-(Hydroxymethyl)glutathione |
|---|
| Description | S-Hydroxymethylglutathione belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. S-Hydroxymethylglutathione exists in all living species, ranging from bacteria to plants to humans. Based on a literature review a significant number of articles have been published on S-Hydroxymethylglutathione. |
|---|
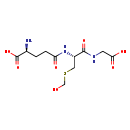
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-amino-4-[1-CARBOXYMETHYL-carbamoyl)-2-hydroxymethylsulfanyl-ethylcarbamoyl]-butyrIC ACID | ChEBI, HMDB | | S-Hydroxymethylglutathione | ChEBI | | 2-amino-4-[1-CARBOXYMETHYL-carbamoyl)-2-hydroxymethylsulfanyl-ethylcarbamoyl]-butyrate | Generator, HMDB | | 2-amino-4-[1-CARBOXYMETHYL-carbamoyl)-2-hydroxymethylsulphanyl-ethylcarbamoyl]-butyrate | Generator, HMDB | | 2-amino-4-[1-CARBOXYMETHYL-carbamoyl)-2-hydroxymethylsulphanyl-ethylcarbamoyl]-butyric acid | Generator, HMDB | | S-Hydroxymethyl-glutathione | HMDB | | SerGSH | MeSH, HMDB | | Formaldehyde-glutathione thiohemiacetal | HMDB | | Glutathione-formaldehyde thiohemiacetal | HMDB | | L-gamma-Glutamyl-S-(hydroxymethyl)-L-cysteinylglycine | HMDB | | L-γ-Glutamyl-S-(hydroxymethyl)-L-cysteinylglycine | HMDB | | S-(Hydroxymethyl)glutathione | HMDB |
|
|---|
| Chemical Formula | C11H19N3O7S |
|---|
| Average Molecular Weight | 337.349 |
|---|
| Monoisotopic Molecular Weight | 337.094370667 |
|---|
| IUPAC Name | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-[(hydroxymethyl)sulfanyl]ethyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | S-hydroxymethylglutathione |
|---|
| CAS Registry Number | 32260-87-0 |
|---|
| SMILES | N[C@@H](CCC(=O)N[C@@H](CSCO)C(=O)NCC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H19N3O7S/c12-6(11(20)21)1-2-8(16)14-7(4-22-5-15)10(19)13-3-9(17)18/h6-7,15H,1-5,12H2,(H,13,19)(H,14,16)(H,17,18)(H,20,21)/t6-,7-/m0/s1 |
|---|
| InChI Key | PIUSLWSYOYFRFR-BQBZGAKWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Gamma-glutamyl alpha peptide
- Glutamine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Dicarboxylic acid or derivatives
- Fatty amide
- Fatty acyl
- Fatty acid
- N-acyl-amine
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Sulfenyl compound
- Carboxylic acid
- Organopnictogen compound
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organic oxide
- Primary amine
- Organosulfur compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01po-6291000000-700a78b68cd3c72dace0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0ukl-9410370000-758e7d3d20ef5e803fa9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00du-0195000000-0fb852c5aa71ebfacbc3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0570-4690000000-74c4fbcadf9e75cea5e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4920000000-a75d217a2989134725ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-5089000000-0963b985ed393a77403b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05di-5393000000-80626db3d68b3e84d33f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fu-9200000000-c770bc62700bd5b4872c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0029000000-9285be543171793a7f61 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-1941000000-3b0ed9e0505deb83df54 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9400000000-cdcded7632edf6274abf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05g0-0193000000-b9ae6fff6991f7a74ac1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-9440000000-4e976999daadff083f1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dl-9400000000-49e525bdebda32941d94 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|