| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:27:34 UTC |
|---|
| Update Date | 2020-05-21 16:28:47 UTC |
|---|
| BMDB ID | BMDB0006915 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
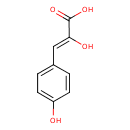
| Common Name | 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid |
|---|
| Description | 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid, also known as 4,alpha-dihydroxycinnamic acid or 4-hydroxy-enol-phenylpyruvate, belongs to the class of organic compounds known as phenylpyruvic acid derivatives. Phenylpyruvic acid derivatives are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid exists in all living organisms, ranging from bacteria to humans. Based on a literature review very few articles have been published on 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,alpha-Dihydroxycinnamic acid | ChEBI | | 4-Hydroxy-enol-phenylpyruvate | Kegg | | 4,a-Dihydroxycinnamate | Generator | | 4,a-Dihydroxycinnamic acid | Generator | | 4,alpha-Dihydroxycinnamate | Generator | | 4,Α-dihydroxycinnamate | Generator | | 4,Α-dihydroxycinnamic acid | Generator | | 4-Hydroxy-enol-phenylpyruvic acid | Generator | | 2-Hydroxy-3-(4-hydroxyphenyl)propenoate | Generator |
|
|---|
| Chemical Formula | C9H8O4 |
|---|
| Average Molecular Weight | 180.1574 |
|---|
| Monoisotopic Molecular Weight | 180.042258744 |
|---|
| IUPAC Name | (2Z)-2-hydroxy-3-(4-hydroxyphenyl)prop-2-enoic acid |
|---|
| Traditional Name | 4,α-dihydroxycinnamic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)C(\O)=C\C1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H8O4/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-5,10-11H,(H,12,13)/b8-5- |
|---|
| InChI Key | GQYBCIHRWMPOOF-YVMONPNESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpyruvic acid derivatives. Phenylpyruvic acid derivatives are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylpyruvic acid derivatives |
|---|
| Direct Parent | Phenylpyruvic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamic acid
- Cinnamic acid or derivatives
- Coumaric acid
- Coumaric acid or derivatives
- Hydroxycinnamic acid
- Hydroxycinnamic acid or derivatives
- Enol-phenylpyruvate
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocarboxylic acid or derivatives
- Enol
- Carboxylic acid
- Carboxylic acid derivative
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-1900000000-10c51d0329cb2a35dbb8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00gi-6259000000-cdf2744454615081e02f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bu0-0900000000-a08730e02c84f43e7926 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-2eb4f2eaa5d547249a3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-7900000000-63f2942443ac7bfdce48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-8576bdecf0b58e989df6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-46e515a63da5ddf92de9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0900000000-3c0a27f480b5bb59093c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-02f0fa9241cf4b1591c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9800000000-0e7b12140d77aa62a512 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0api-4900000000-6f21f2ecdec85109e7d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01qi-0900000000-28cf4812bb35ce056a15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-1900000000-bd40f97e341181046c0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9200000000-c7f40face58730829b4a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|