| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:30:40 UTC |
|---|
| Update Date | 2020-05-21 16:27:39 UTC |
|---|
| BMDB ID | BMDB0007104 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | DG(16:0/18:3(6Z,9Z,12Z)/0:0) |
|---|
| Description | DG(16:0/18:3(6Z,9Z,12Z)/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(16:0/18:3(6Z,9Z,12Z)/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway. |
|---|
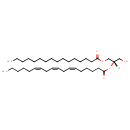
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Diacylglycerol(34:3) | HMDB | | DAG(16:0/18:3) | HMDB | | 1-Palmitoyl-2-g-linolenoyl-sn-glycerol | HMDB | | DG(16:0/18:3) | HMDB | | Diglyceride | HMDB | | Diacylglycerol(16:0/18:3) | HMDB | | DAG(34:3) | HMDB | | Diacylglycerol | HMDB | | DG(34:3) | HMDB | | 1-Hexadecanoyl-2-(6Z,9Z,12Z-octadecatrienoyl)-sn-glycerol | HMDB | | DG(16:0/18:3(6Z,9Z,12Z)/0:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C37H66O5 |
|---|
| Average Molecular Weight | 590.9169 |
|---|
| Monoisotopic Molecular Weight | 590.491025222 |
|---|
| IUPAC Name | (2S)-1-(hexadecanoyloxy)-3-hydroxypropan-2-yl (6Z,9Z,12Z)-octadeca-6,9,12-trienoate |
|---|
| Traditional Name | diacylglycerol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](CO)(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCC\C=C/C\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C37H66O5/c1-3-5-7-9-11-13-15-17-18-20-22-24-26-28-30-32-37(40)42-35(33-38)34-41-36(39)31-29-27-25-23-21-19-16-14-12-10-8-6-4-2/h11,13,17-18,22,24,35,38H,3-10,12,14-16,19-21,23,25-34H2,1-2H3/b13-11-,18-17-,24-22-/t35-/m0/s1 |
|---|
| InChI Key | YFBCIDURFTWKDQ-OGFNIKLTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- 1,2-acyl-sn-glycerol
- Diacylglycerol
- Diradylglycerol
- Fatty acid ester
- Glycerolipid
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03di-4592114000-7193fbe5ec1c0e3e6c72 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("DG(16:0/18:3(6Z,9Z,12Z)/0:0),1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000009000-2c9dba2a539fdca961d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03kr-0009030000-6d3823ea5e5e30c2e278 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g9-0009013000-f02bd6bc82186632d789 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000009000-06c2ad30942913111b60 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000009000-06c2ad30942913111b60 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a50-0009000000-764d62ab96e8196e5e1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p6-2166290000-57e7e4081847392a7bf1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-4393010000-2b244c3a3a3ac136071f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g0-7978000000-229ee169b246396e473a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000009000-418bfe830d05946bacd1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03kr-0009030000-d5126a3094a7234981e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g9-0009013000-9683375c1a7e345b5fc1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-2086090000-ceef029f8a823308bc37 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2091000000-329bcb7f1e2743e90230 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a70-2090000000-8d0287c89dfb4d229dc5 | View in MoNA |
|---|
|
|---|
| Pathways | |
|---|