| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:31:46 UTC |
|---|
| Update Date | 2020-05-21 16:27:45 UTC |
|---|
| BMDB ID | BMDB0007160 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
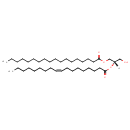
| Common Name | DG(18:0/18:1(9Z)/0:0) |
|---|
| Description | DG(18:0/18:1(9Z)/0:0)[iso2], also known as dg(18:0/18:1(9z)/0:0)[iso2] or DAG(18:0/18:1), belongs to the class of organic compounds known as 1,2-dg(18:0/18:1(9z)/0:0)[iso2]s. These are dg(18:0/18:1(9z)/0:0)[iso2]s containing a glycerol acylated at positions 1 and 2. Thus, DG(18:0/18:1(9Z)/0:0)[iso2] is considered to be a diradylglycerol lipid molecule. DG(18:0/18:1(9Z)/0:0)[iso2] is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. DG(18:0/18:1(9Z)/0:0)[iso2] exists in all living organisms, ranging from bacteria to humans. In cattle, DG(18:0/18:1(9Z)/0:0)[iso2] is involved in a couple of metabolic pathways, which include de novo triacylglycerol biosynthesis TG(18:0/18:1(9Z)/18:1(9Z)) pathway and de novo triacylglycerol biosynthesis TG(18:0/18:1(9Z)/20:0) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Octadecanoyl-2-(9Z-octadecenoyl)-sn-glycerol | ChEBI | | DG (18:0/18:1(N-9)/0:0) | ChEBI | | sn-SODG | MeSH | | DAG(36:1) | Lipid Annotator, HMDB | | Diacylglycerol(36:1) | Lipid Annotator, HMDB | | DAG(18:0/18:1) | Lipid Annotator, HMDB | | Diglyceride | Lipid Annotator, HMDB | | Diacylglycerol(18:0/18:1) | Lipid Annotator, HMDB | | DG(18:0/18:1(9Z)/0:0) | Lipid Annotator | | Diacylglycerol | Lipid Annotator, HMDB | | 1-stearoyl-2-oleoyl-sn-glycerol | Lipid Annotator, HMDB | | DG(18:0/18:1) | Lipid Annotator, HMDB | | DG(36:1) | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula | C39H74O5 |
|---|
| Average Molecular Weight | 623.0019 |
|---|
| Monoisotopic Molecular Weight | 622.553625478 |
|---|
| IUPAC Name | (2S)-1-hydroxy-3-(octadecanoyloxy)propan-2-yl (9Z)-octadec-9-enoate |
|---|
| Traditional Name | α-oleo-β-stearin |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](CO)(COC(=O)CCCCCCCCCCCCCCCCC)OC(=O)CCCCCCC\C=C/CCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C39H74O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(41)43-36-37(35-40)44-39(42)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h18,20,37,40H,3-17,19,21-36H2,1-2H3/b20-18-/t37-/m0/s1 |
|---|
| InChI Key | SAEPUUXWQQNLGN-LVVMQYBKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,2-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-acyl-sn-glycerol
- Fatty acid ester
- Fatty acyl
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-029i-4391446000-98c8b2aac6d36fe7172e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("DG(18:0/18:1(9Z)/0:0),1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000009000-fce9091ab20877af1859 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052o-0009004000-31e141f7dec25acc8594 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-0009004000-8978ea08da2193fc6059 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000009000-bab9e83f09359631bc8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052o-0009004000-9f455557dd938e5b9983 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-0009004000-818182964e61f6d27663 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000009000-8b1291422a70b2640ab8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000009000-8b1291422a70b2640ab8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0009000000-602c71feebdd81b7a4e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1044009000-b06b88971a4a6bb66cbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2093000000-339bdaf2372dfce18ca0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-1191000000-318ba0d71ab598d50362 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-2057109000-807a9d766588bd97d68f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-3296002000-c0fae04dff0f27f2f833 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014r-6796100000-e68d3a4a498df612a02b | View in MoNA |
|---|
|
|---|
| Pathways | |
|---|