| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:33:00 UTC |
|---|
| Update Date | 2020-05-21 16:27:51 UTC |
|---|
| BMDB ID | BMDB0007221 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
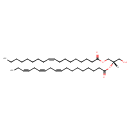
| Common Name | DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0) |
|---|
| Description | DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0)[iso2], also known as DAG(18:1/18:3) or DAG(18:1OMEGA9/18:3OMEGA3), belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. Thus, DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0)[iso2] is considered to be a diradylglycerol lipid molecule. DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0)[iso2] is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. In cattle, DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0)[iso2] is involved in the metabolic pathway called phosphatidylcholine biosynthesis PC(18:1(9Z)/18:3(9Z,12Z,15Z)) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(9Z)-Octadecenoyl-2-(9Z,12Z,15Z)-octadecatrienoyl-sn-glycerol | ChEBI | | 1-(9Z-Octadecenoyl)-2-(9Z,12Z,15Z-octadecatrienoyl)-sn-glycerol | ChEBI | | DAG(18:1/18:3) | ChEBI | | DAG(18:1OMEGA9/18:3OMEGA3) | ChEBI | | DAG(36:4) | ChEBI | | DG(18:1(OMEGA-9)/18:3(OMEGA-3)/0:0) | ChEBI | | DG(18:1/18:3) | ChEBI | | DG(18:1/18:3/0:0) | ChEBI | | DG(18:1OMEGA9/18:3OMEGA3) | ChEBI | | DG(36:4) | ChEBI | | Diacylglycerol(18:1/18:3) | ChEBI | | Diacylglycerol(18:1omega9/18:3omega3) | ChEBI | | Diacylglycerol(36:4) | ChEBI | | 1-Oleoyl-2-a-linolenoyl-sn-glycerol | HMDB | | 1-Oleoyl-2-alpha-linolenoyl-sn-glycerol | HMDB | | DAG(18:1N9/18:3N3) | HMDB | | DAG(18:1W9/18:3W3) | HMDB | | DG(18:1N9/18:3N3) | HMDB | | DG(18:1W9/18:3W3) | HMDB | | Diacylglycerol | HMDB | | Diacylglycerol(18:1n9/18:3n3) | HMDB | | Diacylglycerol(18:1W9/18:3W3) | HMDB | | Diglyceride | HMDB | | 1-(9Z-Octadecenoyl)-2-(9Z,12Z,15Z-octadeatrienoyl)-sn-glycerol | HMDB | | DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0) | Lipid Annotator, ChEBI |
|
|---|
| Chemical Formula | C39H68O5 |
|---|
| Average Molecular Weight | 616.9542 |
|---|
| Monoisotopic Molecular Weight | 616.506675286 |
|---|
| IUPAC Name | (2S)-1-hydroxy-3-[(9Z)-octadec-9-enoyloxy]propan-2-yl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate |
|---|
| Traditional Name | diacylglycerol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](CO)(COC(=O)CCCCCCC\C=C/CCCCCCCC)OC(=O)CCCCCCC\C=C/C\C=C/C\C=C/CC |
|---|
| InChI Identifier | InChI=1S/C39H68O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(41)43-36-37(35-40)44-39(42)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h6,8,12,14,17-20,37,40H,3-5,7,9-11,13,15-16,21-36H2,1-2H3/b8-6-,14-12-,19-17-,20-18-/t37-/m0/s1 |
|---|
| InChI Key | YAKOEWASAGCYLB-VGCWMEDESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- 1,2-acyl-sn-glycerol
- Diacylglycerol
- Diradylglycerol
- Fatty acid ester
- Glycerolipid
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-022i-5490303000-793276446ffd91f9712f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("DG(18:1(9Z)/18:3(9Z,12Z,15Z)/0:0),1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000009000-0033c71a807b4af9c6cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0009031000-c5b3ed85f2ba611d2ac4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0009003000-0d2873754b46c8e947ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-2175289000-04faa597ebf2c6e41903 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-4196130000-7cdb05783272593dcf29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-3397000000-7c2ca5adbba1e8a88dd6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000009000-7da2b68332eda96bc7dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0009031000-cc19d93a45673d2b340d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0009003000-1c0b17eaf678bb8c3420 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000009000-c5201b03fd289f8bd404 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000009000-c5201b03fd289f8bd404 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-0009000000-b1f46be74522984ed59e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2075009000-5b3b223d4bc2ac6e103d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01z9-4094000000-68f9a28cb4c3e74910be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-1090000000-34baf4f0157b4b888332 | View in MoNA |
|---|
|
|---|
| Pathways | |
|---|