| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:36:42 UTC |
|---|
| Update Date | 2020-05-21 16:28:00 UTC |
|---|
| BMDB ID | BMDB0007406 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
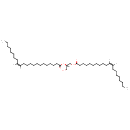
| Common Name | DG(20:1(11Z)/22:1(13Z)/0:0) |
|---|
| Description | DG(20:1(11Z)/22:1(13Z)/0:0), also known as DAG(20:1/22:1) or diacylglycerol(20:1/22:1), belongs to the class of organic compounds known as 1,2-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 2. Thus, DG(20:1(11Z)/22:1(13Z)/0:0) is considered to be a diradylglycerol lipid molecule. DG(20:1(11Z)/22:1(13Z)/0:0) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. DG(20:1(11Z)/22:1(13Z)/0:0) participates in a number of enzymatic reactions, within cattle. In particular, DG(20:1(11Z)/22:1(13Z)/0:0) can be biosynthesized from PA(20:1(11Z)/22:1(13Z)) through its interaction with the enzyme phosphatidate phosphatase. Furthermore, DG(20:1(11Z)/22:1(13Z)/0:0) and docosadienoyl-CoA can be converted into TG(20:1(11Z)/22:1(13Z)/22:2(13Z,16Z)); which is mediated by the enzyme diacylglycerol O-acyltransferase. Furthermore, DG(20:1(11Z)/22:1(13Z)/0:0) can be biosynthesized from PA(20:1(11Z)/22:1(13Z)) through the action of the enzyme phosphatidate phosphatase. Finally, DG(20:1(11Z)/22:1(13Z)/0:0) and tetracosanoyl-CoA can be converted into TG(20:1(11Z)/22:1(13Z)/24:0); which is catalyzed by the enzyme diacylglycerol O-acyltransferase. In cattle, DG(20:1(11Z)/22:1(13Z)/0:0) is involved in a couple of metabolic pathways, which include de novo triacylglycerol biosynthesis TG(20:1(11Z)/22:1(13Z)/22:2(13Z,16Z)) pathway and de novo triacylglycerol biosynthesis TG(20:1(11Z)/22:1(13Z)/24:0) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| DAG(20:1/22:1) | HMDB | | 1-Eicosenoyl-2-erucoyl-sn-glycerol | HMDB | | DAG(42:2) | HMDB | | Diglyceride | HMDB | | Diacylglycerol(20:1/22:1) | HMDB | | DG(42:2) | HMDB | | Diacylglycerol | HMDB | | DG(20:1/22:1) | HMDB | | Diacylglycerol(42:2) | HMDB | | 1-(11-Eicosenoyl)-2-(13Z-docosenoyl)-sn-glycerol | HMDB | | DG(20:1(11Z)/22:1(13Z)/0:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C45H84O5 |
|---|
| Average Molecular Weight | 705.1455 |
|---|
| Monoisotopic Molecular Weight | 704.631875798 |
|---|
| IUPAC Name | (2S)-1-hydroxy-3-[(11Z)-icos-11-enoyloxy]propan-2-yl (13Z)-docos-13-enoate |
|---|
| Traditional Name | diacylglycerol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(CCCCCCCC)=C(/[H])CCCCCCCCCCCC(=O)O[C@@]([H])(CO)COC(=O)CCCCCCCCC\C([H])=C(\[H])CCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C45H84O5/c1-3-5-7-9-11-13-15-17-19-21-22-24-26-28-30-32-34-36-38-40-45(48)50-43(41-46)42-49-44(47)39-37-35-33-31-29-27-25-23-20-18-16-14-12-10-8-6-4-2/h17-20,43,46H,3-16,21-42H2,1-2H3/b19-17-,20-18-/t43-/m0/s1 |
|---|
| InChI Key | TXSPVVDGQJIPFG-ALXJKLDESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,2-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-acyl-sn-glycerol
- Fatty acid ester
- Fatty acyl
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000900-f57912e5a3115d4646fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014t-0009003100-ed6450d3613f5f52d018 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01bb-0009000300-77999fb1251df0539e4f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000000900-22a8e38c4a96cde10ac8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000000900-22a8e38c4a96cde10ac8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0150-0009600100-ce97e5dbcde58cb35030 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0129112600-4085718b7b5780e4a9ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06dl-0169000000-e0f2cc21a5b425579e74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0597-6913000000-2cd5f8fd896caedc1264 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000900-46bb7b8edeb864cb1e02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014t-0009003100-7159e798dcce034976a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01bb-0009000300-e80280fd3254f006dc7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0009000300-aa24a8f5220131c7d267 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ap3-2019000100-31184bacac4329bdb91e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-2039000000-dbb0247218b0378083bf | View in MoNA |
|---|
|
|---|
| Pathways | |
|---|