| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:46:45 UTC |
|---|
| Update Date | 2020-06-04 19:10:28 UTC |
|---|
| BMDB ID | BMDB0007903 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
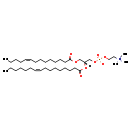
| Common Name | PC(14:1(9Z)/16:1(9Z)) |
|---|
| Description | PC(14:1(9Z)/16:1(9Z)), also known as PC(30:2) or pc(14:1(9z)/16:1(9z)), belongs to the class of organic compounds known as phosphatidylcholines. These are glycerophosphocholines in which the two free -OH are attached to one fatty acid each through an ester linkage. Thus, PC(14:1(9Z)/16:1(9Z)) is considered to be a glycerophosphocholine lipid molecule. PC(14:1(9Z)/16:1(9Z)) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. PC(14:1(9Z)/16:1(9Z)) exists in all eukaryotes, ranging from yeast to humans. PC(14:1(9Z)/16:1(9Z)) participates in a number of enzymatic reactions, within cattle. In particular, S-Adenosylhomocysteine and PC(14:1(9Z)/16:1(9Z)) can be biosynthesized from S-adenosylmethionine and pe-nme2(14:1(9Z)/16:1(9Z)); which is mediated by the enzyme phosphatidylethanolamine N-methyltransferase. Furthermore, Cytidine monophosphate and PC(14:1(9Z)/16:1(9Z)) can be biosynthesized from CDP-choline and DG(14:1(9Z)/16:1(9Z)/0:0) through the action of the enzyme choline/ethanolaminephosphotransferase. Finally, PC(14:1(9Z)/16:1(9Z)) and L-serine can be converted into choline and PS(14:1(9Z)/16:1(9Z)) through the action of the enzyme phosphatidylserine synthase. In cattle, PC(14:1(9Z)/16:1(9Z)) is involved in a couple of metabolic pathways, which include phosphatidylcholine biosynthesis PC(14:1(9Z)/16:1(9Z)) pathway and phosphatidylethanolamine biosynthesis pe(14:1(9Z)/16:1(9Z)) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Phosphatidylcholine(14:1/16:1) | HMDB | | PC(30:2) | HMDB | | 1-Myristoleoyl-2-palmitoleoyl-sn-glycero-3-phosphocholine | HMDB | | Lecithin | HMDB | | GPCho(30:2) | HMDB | | GPCho(14:1/16:1) | HMDB | | Phosphatidylcholine(30:2) | HMDB | | 1-(9Z-Tetradecenoyl)-2-(9Z-hexadecenoyl)-sn-glycero-3-phosphocholine | HMDB | | PC(14:1/16:1) | HMDB | | PC(14:1(9Z)/16:1(9Z)) | Lipid Annotator |
|
|---|
| Chemical Formula | C38H72NO8P |
|---|
| Average Molecular Weight | 701.9539 |
|---|
| Monoisotopic Molecular Weight | 701.499554797 |
|---|
| IUPAC Name | (2-{[(2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-[(9Z)-tetradec-9-enoyloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | lecithin |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCC\C=C/CCCCCCCC(=O)O[C@]([H])(COC(=O)CCCCCCC\C=C/CCCC)COP([O-])(=O)OCC[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C38H72NO8P/c1-6-8-10-12-14-16-18-19-21-23-25-27-29-31-38(41)47-36(35-46-48(42,43)45-33-32-39(3,4)5)34-44-37(40)30-28-26-24-22-20-17-15-13-11-9-7-2/h13,15-16,18,36H,6-12,14,17,19-35H2,1-5H3/b15-13-,18-16-/t36-/m1/s1 |
|---|
| InChI Key | RUTXZOOOUKHIRR-KQGJYXFZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylcholines. These are glycerophosphocholines in which the two free -OH are attached to one fatty acid each through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | Phosphatidylcholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organic salt
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-0000915000-70cc220ef0e06e106fa6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zi0-0090000200-a647583b7468aca22802 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0190000000-4a412e5f8d73402c5ed6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-4290000000-03989d55493c73536a31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000900-5e86fac3277edd257bad | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000001900-d689ed047e4811394499 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-0700691100-b1b962c108a0c0b79340 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-015b7563f35058194e4b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0030000900-fd2ae918b9bd28bf4ae8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uii-0090000400-cd9d490ca073d32c9e59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000900-c0264bc5a3658a84089e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000001900-f99701a4c0dc029e939b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fis-0101791100-07091dabbadf7fa8ebb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000900-c0783fc627a546653f44 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090042700-0c9a81041296cc2790ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-3394000000-397dae97836f53018b88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000000900-1fd7bbea2430b811e0ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0600000900-4b1c66063a5797985307 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1900412100-d5b06fcc3b23dd902711 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|