| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:22:13 UTC |
|---|
| Update Date | 2020-05-21 16:28:49 UTC |
|---|
| BMDB ID | BMDB0011644 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
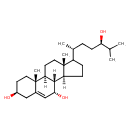
| Common Name | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol |
|---|
| Description | (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. Based on a literature review a significant number of articles have been published on (24R)-Cholest-5-ene-3-beta,7-alpha,24-triol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (24R)-Cholest-5-ene-3-b,7-a,24-triol | Generator | | (24R)-Cholest-5-ene-3-β,7-α,24-triol | Generator | | (24R)-Cholest-5-ene 3-b,7-a,24-triol | HMDB | | (24R)-Cholest-5-ene-3beta,7alpha,24-triol | HMDB | | 5a-Cholesta-7,24-dien-3-b-ol | HMDB | | 5a-Cholesta-7,24-dien-3-beta-ol | HMDB |
|
|---|
| Chemical Formula | C27H46O3 |
|---|
| Average Molecular Weight | 418.6523 |
|---|
| Monoisotopic Molecular Weight | 418.344695338 |
|---|
| IUPAC Name | (1S,2R,5S,9S,10S,11S,15R)-14-[(2R,5R)-5-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,9-diol |
|---|
| Traditional Name | (1S,2R,5S,9S,10S,11S,15R)-14-[(2R,5R)-5-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,9-diol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CCC([C@H](C)CC[C@@H](O)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)C=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H46O3/c1-16(2)23(29)9-6-17(3)20-7-8-21-25-22(11-13-27(20,21)5)26(4)12-10-19(28)14-18(26)15-24(25)30/h15-17,19-25,28-30H,6-14H2,1-5H3/t17-,19+,20?,21+,22+,23-,24-,25+,26+,27-/m1/s1 |
|---|
| InChI Key | ZNCHPOYZMVVJCK-DPFMVWRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trihydroxy bile acid, alcohol, or derivatives
- 24-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 7-hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-1239400000-cd1b8c75eaf66591d8c2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00xu-2110149000-138cf2097993192ea364 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0007900000-17814845dbf0bec54cfd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-3009400000-fd95622ed3f4f1fae1c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00fr-5029000000-70e1fb26560a91668d82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0005900000-a892257c0a1e6d75d1d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-0009500000-66d60259ffcab13684b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fl9-7009200000-99af00a0dfd9097d149b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-f2cb6399034352e924c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1002900000-b6143eba8eae9ed9668b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014j-3019800000-4bfcf5c6acaa256d7599 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zgi-0308900000-66129b219db1313f7273 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-3329100000-91ec216cab7fcf837baf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0adi-3943000000-12a20d116574d822ddef | View in MoNA |
|---|
|
|---|