| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:37:16 UTC |
|---|
| Update Date | 2020-05-21 16:28:19 UTC |
|---|
| BMDB ID | BMDB0012380 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
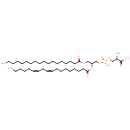
| Common Name | PS(18:0/18:2(9Z,12Z)) |
|---|
| Description | PS(18:0/18:2(9Z,12Z)), also known as PS(18:0/18:2) or pSer(36:2), belongs to the class of organic compounds known as phosphatidylserines. These are glycerophosphoserines in which two fatty acids are bonded to the glycerol moiety through ester linkages. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. Thus, PS(18:0/18:2(9Z,12Z)) is considered to be a glycerophosphoserine lipid molecule. PS(18:0/18:2(9Z,12Z)) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. PS(18:0/18:2(9Z,12Z)) participates in a number of enzymatic reactions, within cattle. In particular, Choline and PS(18:0/18:2(9Z,12Z)) can be biosynthesized from PC(18:0/18:2(9Z,12Z)) and L-serine through its interaction with the enzyme phosphatidylserine synthase. Furthermore, PS(18:0/18:2(9Z,12Z)) can be converted into PE(18:0/18:2(9Z,12Z)) through its interaction with the enzyme phosphatidylserine decarboxylase. Finally, PS(18:0/18:2(9Z,12Z)) can be converted into PE(18:0/18:2(9Z,12Z)) through the action of the enzyme phosphatidylserine decarboxylase. In cattle, PS(18:0/18:2(9Z,12Z)) is involved in a couple of metabolic pathways, which include phosphatidylethanolamine biosynthesis pe(18:0/18:2(9Z,12Z)) pathway and phosphatidylcholine biosynthesis PC(18:0/18:2(9Z,12Z)) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Octadecanoyl-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phospho-L-serine | ChEBI | | 1-Octadecanoyl-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphoserine | ChEBI | | 1-Stearoyl-2-linoleoyl-sn-glycero-3-phosphoserine | ChEBI | | Phosphatidylserine(18:0/18:2) | ChEBI | | Phosphatidylserine(18:0/18:2n6) | ChEBI | | Phosphatidylserine(18:0/18:2W6) | ChEBI | | PS(18:0/18:2) | ChEBI | | PS(18:0/18:2N6) | ChEBI | | pSer(18:0/18:2) | ChEBI | | pSer(18:0/18:2n6) | ChEBI | | pSer(18:0/18:2W6) | ChEBI | | pSer(36:2) | ChEBI | | Phosphatidylserine(36:2) | HMDB | | PS(18:0/18:2W6) | HMDB | | PS(36:2) | HMDB | | PS(18:0/18:2(9Z,12Z)) | Lipid Annotator | | 1-Stearoyl-2-linoleoyl-GPS | HMDB | | Phosphatidylserine(18:0/18:2(9Z,12Z)) | HMDB | | Pser(18:0/18:2(9Z,12Z)) | HMDB |
|
|---|
| Chemical Formula | C42H78NO10P |
|---|
| Average Molecular Weight | 788.0432 |
|---|
| Monoisotopic Molecular Weight | 787.536334233 |
|---|
| IUPAC Name | (2S)-2-amino-3-({hydroxy[(2R)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]-3-(octadecanoyloxy)propoxy]phosphoryl}oxy)propanoic acid |
|---|
| Traditional Name | (2S)-2-amino-3-{[hydroxy((2R)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]-3-(octadecanoyloxy)propoxy)phosphoryl]oxy}propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C42H78NO10P/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-40(44)50-35-38(36-51-54(48,49)52-37-39(43)42(46)47)53-41(45)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h12,14,18,20,38-39H,3-11,13,15-17,19,21-37,43H2,1-2H3,(H,46,47)(H,48,49)/b14-12-,20-18-/t38-,39+/m1/s1 |
|---|
| InChI Key | AGTPCXBHIGMTEU-NREGDSCDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylserines. These are glycerophosphoserines in which two fatty acids are bonded to the glycerol moiety through ester linkages. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoserines |
|---|
| Direct Parent | Phosphatidylserines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacyl-glycerol-3-phosphoserine
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Tricarboxylic acid or derivatives
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Amino acid
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Carboxylic acid
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Carbonyl group
- Organic oxide
- Primary amine
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-9030020400-35fdc502a0c5332d80bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000f-9020000000-6e39fb542c07d3f7f70a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9033011100-8e765ae1c978794e0a1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0159-1190021200-cc18197b14e3a7318b10 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00o0-5290010000-2cba48fff257e2b3d121 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9130000000-5fbab6d31a1f06ef3f89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-14i6-0000004900-ef7e44d1be3adfa5a09d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k96-0400004900-31bf8f1446ce1d1b222f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-0400004900-31bf8f1446ce1d1b222f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000001900-5dedc46c40b917f10744 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0002059500-fc569869be28f3940fd8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0002059100-44bc5c73d7a48a8f8fb5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-a24428b88fc31234d1d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000001900-9c4fec99b40594066f80 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-017s-0070905300-7b5d83873dd2a79e2296 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03k9-0000001690-430793a1f49553bdd03e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000290-2af557577adf8dd4d0b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0090003310-ad089ba119dae26a0d13 | View in MoNA |
|---|
|
|---|