| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:38:57 UTC |
|---|
| Update Date | 2020-05-21 16:28:49 UTC |
|---|
| BMDB ID | BMDB0012458 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7 alpha-Hydroxy-3-oxo-4-cholestenoate |
|---|
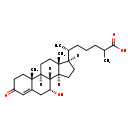
| Description | 7alpha-Hydroxy-3-oxo-4-cholestenoate, also known as 7-hoca or (7α)-7-hydroxy-3-oxocholest-4-en-26-Oate, belongs to the class of organic compounds known as monohydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or any of their derivatives bearing a hydroxyl group. Based on a literature review a significant number of articles have been published on 7alpha-Hydroxy-3-oxo-4-cholestenoate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7-Hoca | ChEBI | | 7a-Hydroxy-3-oxo-4-cholestenoate | Generator | | 7a-Hydroxy-3-oxo-4-cholestenoic acid | Generator | | 7alpha-Hydroxy-3-oxo-4-cholestenoic acid | Generator | | 7Α-hydroxy-3-oxo-4-cholestenoate | Generator | | 7Α-hydroxy-3-oxo-4-cholestenoic acid | Generator | | 7 alpha-Hydroxy-3-oxo-4-cholestenoic acid | HMDB | | (7Α)-7-hydroxy-3-oxocholest-4-en-26-Oic acid | HMDB | | (7Α)-7-hydroxy-3-oxocholest-4-en-26-Oate | HMDB | | 7Α-hydroxy-3-oxocholest-4-en-26-Oic acid | HMDB | | (7alpha)-7-Hydroxy-3-oxocholest-4-en-26-Oic acid | HMDB | | (7alpha)-7-Hydroxy-3-oxocholest-4-en-26-Oate | HMDB | | 7alpha-Hydroxy-3-oxocholest-4-en-26-Oic acid | HMDB | | 7-alpha-Hydroxy-3-oxo-4-cholestenoate | HMDB | | 7alpha-Hydroxy-3-oxo-4-cholestenoate | HMDB, Generator |
|
|---|
| Chemical Formula | C27H42O4 |
|---|
| Average Molecular Weight | 430.629 |
|---|
| Monoisotopic Molecular Weight | 430.308309832 |
|---|
| IUPAC Name | (6R)-6-[(1S,2R,9R,10S,11S,14R,15R)-9-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl]-2-methylheptanoic acid |
|---|
| Traditional Name | (6R)-6-[(1S,2R,9R,10S,11S,14R,15R)-9-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl]-2-methylheptanoic acid |
|---|
| CAS Registry Number | 115538-85-7 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)CC4=CC(=O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C27H42O4/c1-16(6-5-7-17(2)25(30)31)20-8-9-21-24-22(11-13-27(20,21)4)26(3)12-10-19(28)14-18(26)15-23(24)29/h14,16-17,20-24,29H,5-13,15H2,1-4H3,(H,30,31)/t16-,17?,20-,21+,22+,23-,24+,26+,27-/m1/s1 |
|---|
| InChI Key | SATGKQGFUDXGAX-MYWFJNCASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monohydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or any of their derivatives bearing a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Monohydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monohydroxy bile acid, alcohol, or derivatives
- Steroid acid
- 3-oxosteroid
- 3-oxo-delta-4-steroid
- 7-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Medium-chain fatty acid
- Cyclohexenone
- Hydroxy fatty acid
- Fatty acyl
- Cyclic alcohol
- Cyclic ketone
- Secondary alcohol
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0005900000-b7bca15cabd9e12c6696 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-029j-1009300000-55f0578d6b1582318cfc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-3219000000-f33373cace65dac79a13 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0001900000-ad4ad49ae3c3a393e0e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02dr-0007900000-88a170ea510b330ee78d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avi-6009200000-78baf4ea8fd63c360662 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-2c11db7f4225ffd58c75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0007900000-319ad83ee01108cd0a35 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2003900000-183aa10ccc1b913cc96d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0004900000-6dd1885d6034e79dff91 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-3289300000-c62c18e4c41396c10579 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059j-5971000000-425901eed2cf0114ca5a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|