| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:38:58 UTC |
|---|
| Update Date | 2020-05-21 16:28:49 UTC |
|---|
| BMDB ID | BMDB0012459 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7 alpha,26-Dihydroxy-4-cholesten-3-one |
|---|
| Description | 7 alpha,26-Dihydroxy-4-cholesten-3-one belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. Based on a literature review a small amount of articles have been published on 7 alpha,26-Dihydroxy-4-cholesten-3-one. |
|---|
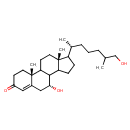
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7 a,26-Dihydroxy-4-cholesten-3-one | Generator | | 7 Α,26-dihydroxy-4-cholesten-3-one | Generator | | (7alpha)-7,26-Dihydroxy-cholest-4-en-3-one | HMDB | | 4-Cholesten-7alpha,26-diol-3-one | HMDB | | 7,26-Dhxyclso | HMDB, MeSH | | 7alpha,26-Dihydroxy-4-cholesten-3-one | HMDB | | 7alpha,26-Dihydroxycholest-4-en-3-one | HMDB |
|
|---|
| Chemical Formula | C27H44O3 |
|---|
| Average Molecular Weight | 416.6365 |
|---|
| Monoisotopic Molecular Weight | 416.329045274 |
|---|
| IUPAC Name | (2R,9R,15R)-9-hydroxy-14-[(2R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | (2R,9R,15R)-9-hydroxy-14-[(2R)-7-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| CAS Registry Number | 4675-38-1 |
|---|
| SMILES | CC(CO)CCC[C@@H](C)C1CCC2C3[C@H](O)CC4=CC(=O)CC[C@]4(C)C3CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H44O3/c1-17(16-28)6-5-7-18(2)21-8-9-22-25-23(11-13-27(21,22)4)26(3)12-10-20(29)14-19(26)15-24(25)30/h14,17-18,21-25,28,30H,5-13,15-16H2,1-4H3/t17?,18-,21?,22?,23?,24-,25?,26+,27-/m1/s1 |
|---|
| InChI Key | KVJVJJWIEXCECB-JZGXDVPNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - 26-hydroxysteroid
- Dihydroxy bile acid, alcohol, or derivatives
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 7-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Fatty alcohol
- Cyclohexenone
- Fatty acyl
- Cyclic alcohol
- Secondary alcohol
- Cyclic ketone
- Ketone
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-0359200000-c5e766bc8da3445acdea | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0002-1220390000-2ea93ad32d390af352da | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0009200000-f13064ffc70da2c2b57d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00l2-1009000000-f344cdc48d7b49f22d6f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-3129000000-b6c48651e7e9ac09edbc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0004900000-7754503899cd9db211c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0009600000-54c6b067e6490ab83b85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0674-2009000000-ceacf291ac96437f5944 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0006900000-1bab66a6ddf2f8b577e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07wj-5749100000-fb55e94ae37f95aa32a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9850000000-4478cbe8a6ea3e417fe2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-67ea14fb7a26b9b2d929 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0005900000-37c9645751a2f52b7424 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0004900000-ca04c33318a7e2c042bc | View in MoNA |
|---|
|
|---|