| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:18:53 UTC |
|---|
| Update Date | 2020-06-04 20:59:41 UTC |
|---|
| BMDB ID | BMDB0000043 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Betaine |
|---|
| Description | Betaine, also known as Bet or acidin pepsin, belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). Betaine exists as a solid, possibly soluble (in water), and a moderately acidic compound (based on its pKa) molecule. Betaine exists in all eukaryotes, ranging from yeast to humans. Betaine participates in a number of enzymatic reactions, within cattle. In particular, Betaine can be biosynthesized from betaine aldehyde; which is catalyzed by the enzyme Alpha-aminoadipic semialdehyde dehydrogenase. In addition, Betaine and homocysteine can be converted into dimethylglycine and L-methionine; which is catalyzed by the enzyme betaine--homocysteine S-methyltransferase 1. In cattle, betaine is involved in the metabolic pathway called the betaine metabolism pathway. |
|---|
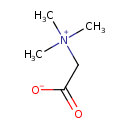
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (Trimethylammoniumyl)acetate | ChEBI | | 1-Carboxy-N,N,N-trimethylmethanaminium inner salt | ChEBI | | 2-N,N,N-Trimethylammonio acetate | ChEBI | | Abromine | ChEBI | | Acidol | ChEBI | | Bet | ChEBI | | N,N,N-Trimethylammonioacetate | ChEBI | | N,N,N-Trimethylglycine | ChEBI | | Trimethylaminoacetate | ChEBI | | Trimethylammonioacetate | ChEBI | | Trimethylglycine | ChEBI | | Trimethylglycocoll | ChEBI | | Glycine betaine | Kegg | | Cystadane | Kegg | | (Trimethylammoniumyl)acetic acid | Generator | | 2-N,N,N-Trimethylammonio acetic acid | Generator | | N,N,N-Trimethylammonioacetic acid | Generator | | Trimethylaminoacetic acid | Generator | | Trimethylammonioacetic acid | Generator | | (Carboxymethyl)trimethylammonium hydroxide inner salt | HMDB | | (Trimethylammonio)acetate | HMDB | | 1-Carboxy-N,N,N-trimethyl-methanaminium | HMDB | | 1-Carboxy-N,N,N-trimethyl-methanaminium hydroxide | HMDB | | a-Earleine | HMDB | | alpha-Earleine | HMDB | | Aminocoat | HMDB | | Betafin | HMDB | | Betafin BCR | HMDB | | Betafin BP | HMDB | | Ektasolve ee | HMDB | | FinnStim | HMDB | | Glycocoll betaine | HMDB | | Glycylbetaine | HMDB | | Greenstim | HMDB | | Loramine amb 13 | HMDB | | Loramine amb-13 | HMDB | | Lycine | HMDB | | Oxyneurine | HMDB | | Rubrine C | HMDB | | Trimethylbetaine glycine | HMDB | | Acidin pepsin | HMDB | | Beaufour brand OF betaine citrate | HMDB | | Byk brand OF betaine phosphate | HMDB | | Fournier brand OF betaine ascorbate and hydrate | HMDB | | Hydrochloride, betaine | HMDB | | Logeais brand OF betaine cyclobutyrate | HMDB | | Novobetaine | HMDB | | Betaine hydrochloride | HMDB | | Betaine orphan brand | HMDB | | Boizot brand OF betaine aspartate | HMDB | | C.B.B. | HMDB | | Citrate de bétaïne upsa | HMDB | | Scorbo-bétaïne | HMDB | | Stea-16 | HMDB | | Stea16 | HMDB | | Acidin-pepsin | HMDB | | Citrate de bétaïne beaufour | HMDB | | Orphan brand OF betaine | HMDB | | Scorbo bétaïne | HMDB | | AcidinPepsin | HMDB | | Betaine, glycine | HMDB | | Hepastyl | HMDB | | Scorbobétaïne | HMDB | | UPSA brand OF betaine citrate | HMDB | | Stea 16 | HMDB | | Betaine | ChEBI |
|
|---|
| Chemical Formula | C5H11NO2 |
|---|
| Average Molecular Weight | 117.1463 |
|---|
| Monoisotopic Molecular Weight | 117.078978601 |
|---|
| IUPAC Name | 2-(trimethylazaniumyl)acetate |
|---|
| Traditional Name | (trimethylammonio)acetate |
|---|
| CAS Registry Number | 107-43-7 |
|---|
| SMILES | C[N+](C)(C)CC([O-])=O |
|---|
| InChI Identifier | InChI=1S/C5H11NO2/c1-6(2,3)4-5(7)8/h4H2,1-3H3 |
|---|
| InChI Key | KWIUHFFTVRNATP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Carboxylic acid salt
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organopnictogen compound
- Hydrocarbon derivative
- Organic salt
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 293 - 301 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 611.0 mg/mL at 19 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fu-9200000000-96ab6e2136fe7e03b63a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-0900000000-feacc600820771d2a77b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-9000000000-e5bbda9fb66994576062 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4i-9000000000-c382759eab7d0a87ac27 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-014i-0900000000-a7beb42e6944181ae5d9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-014i-1900000000-77fbc4a2b76030d53644 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0a4i-9100000000-2941e4997f202293bf71 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a4i-9000000000-9b9e3cecb77ed4b40b7c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0a4i-9000000000-27bbb5f9642306216a0b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-014i-1900000000-82a41cbb0f697910b577 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Positive | splash10-0aor-9500000000-9a0f1853188052d798c6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-066r-5900000000-a6291f86046f32ad3c81 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9200000000-709208d6782f1b588501 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014i-0900000000-a7beb42e6944181ae5d9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014i-1900000000-77fbc4a2b76030d53644 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9100000000-2941e4997f202293bf71 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9000000000-a2560fc2add75f79eadb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0a4i-9000000000-27bbb5f9642306216a0b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0900000000-b45353825d98488d752a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , positive | splash10-0aor-9500000000-3fdd9d17a169db5c58dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-493791dfdd428fc3bd6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-3900000000-b2afc99d27edd18d7f29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0lk9-9500000000-5c3747866d41ab8df618 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-5cf478650fcc3a71b790 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-86832153dde465116f78 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066r-9600000000-2e90f09f2c87dfc45640 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0a4l-9000000000-f32989f795eeceb8b692 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 1H]-TOCSY 2D NMR Spectrum (experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| General References | - Klein MS, Almstetter MF, Schlamberger G, Nurnberger N, Dettmer K, Oefner PJ, Meyer HH, Wiedemann S, Gronwald W: Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J Dairy Sci. 2010 Apr;93(4):1539-50. doi: 10.3168/jds.2009-2563. [PubMed:20338431 ]
- O'Callaghan TF, Vazquez-Fresno R, Serra-Cayuela A, Dong E, Mandal R, Hennessy D, McAuliffe S, Dillon P, Wishart DS, Stanton C, Ross RP: Pasture Feeding Changes the Bovine Rumen and Milk Metabolome. Metabolites. 2018 Apr 6;8(2). pii: metabo8020027. doi: 10.3390/metabo8020027. [PubMed:29642378 ]
- A. Foroutan et al. (2019). A. Foroutan et al. The Chemical Composition of Commercial Cow's Milk (in preparation). Journal of Agricultural and Food Chemistry.
|
|---|