| Synonyms | | Value | Source |
|---|
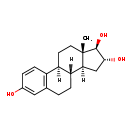
| (16alpha,17beta)-Estra-1,3,5(10)-triene-3,16,17-triol | ChEBI | | 1,3,5(10)-Estratriene-3,16-alpha,17beta-triol | ChEBI | | 16alpha,17beta-Estriol | ChEBI | | 16alpha-Hydroxyestradiol | ChEBI | | 3,16alpha,17beta-Trihydroxy-Delta(1,3,5)-estratriene | ChEBI | | Deuslon-a | ChEBI | | Estriel | ChEBI | | Oestriol | ChEBI | | Trihydroxyestrin | ChEBI | | (16a,17b)-Estra-1,3,5(10)-triene-3,16,17-triol | Generator | | (16Α,17β)-estra-1,3,5(10)-triene-3,16,17-triol | Generator | | 1,3,5(10)-Estratriene-3,16-a,17b-triol | Generator | | 1,3,5(10)-Estratriene-3,16-α,17β-triol | Generator | | 16a,17b-Estriol | Generator | | 16Α,17β-estriol | Generator | | 16a-Hydroxyestradiol | Generator | | 16Α-hydroxyestradiol | Generator | | 3,16a,17b-Trihydroxy-delta(1,3,5)-estratriene | Generator | | 3,16Α,17β-trihydroxy-δ(1,3,5)-estratriene | Generator | | 3,16a,17b-Trihydroxy-δ(1,3,5)-estratriene | HMDB | | 13b-Methyl-1,3,5(10)-gonatriene-3,16a,17b-triol | HMDB | | 16-alpha,17-beta-Estriol | HMDB | | 16-alpha,17-beta-Oestriol | HMDB | | 16-alpha-Hydroxyestradiol | HMDB | | 16a-Estriol | HMDB | | 16alpha,17beta-Oestriol | HMDB | | 16alpha-Hydroxy-17beta-estradiol | HMDB | | 16alpha-Hydroxyoestradiol | HMDB | | 3,16a,17b-Estriol | HMDB | | 3,16a,17b-Trihydroxyestra-1,3,5(10)-triene | HMDB | | 3,16alpha,17beta-Trihydroxy-1,3,5(10)-estratriene | HMDB | | 3,16alpha,17beta-Trihydroxyestra-1,3,5(10)-triene | HMDB | | Aacifemine | HMDB | | Colpogyn | HMDB | | Colpovister | HMDB | | Destriol | HMDB | | Deuslon a | HMDB | | Estra-1,3,5(10)-triene-3,16a,17b-triol | HMDB | | Estratriol | HMDB | | Follicular hormone hydrate | HMDB | | Gynasan | HMDB | | Hemostyptanon | HMDB | | Holin V | HMDB | | Hormomed | HMDB | | Incurin | HMDB | | Klimax e | HMDB | | Klimoral | HMDB | | Oekolp | HMDB | | Oestratriol | HMDB | | Oestriolum | HMDB | | Orestin | HMDB | | Orgastyptin | HMDB | | Ortho-gynest | HMDB | | Ovesterin | HMDB | | Ovestin | HMDB | | Ovestinon | HMDB | | Ovestrion | HMDB | | Ovo-vinces | HMDB | | Theelol | HMDB | | Tridestrin | HMDB | | Triovex | HMDB | | 16-alpha-Hydroxy-estradiol | HMDB | | (16beta,17beta)-Estra-1,3,5(10)-triene-3,16,17-triol | HMDB | | 16beta Hydroxy estradiol | HMDB | | 16beta-Hydroxy-estradiol | HMDB | | Epiestriol | HMDB | | 16 alpha Hydroxy estradiol | HMDB | | 16alpha,17beta Estriol | HMDB | | Estra-1,3,5(10)-triene-3,16beta,17beta-triol | HMDB | | 13beta-Methyl-1,3,5(10)-gonatriene-3,16alpha,17beta-triol | HMDB | | 13β-Methyl-1,3,5(10)-gonatriene-3,16α,17β-triol | HMDB | | 16alpha-Estriol | HMDB | | 16α-Estriol | HMDB | | 3,16alpha,17beta-Estriol | HMDB | | 3,16α,17β-Estriol | HMDB | | 3,16α,17β-Trihydroxyestra-1,3,5(10)-triene | HMDB | | Estriol | HMDB |
|
|---|
| InChI Identifier | InChI=1S/C18H24O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-17,19-21H,2,4,6-7,9H2,1H3/t13-,14-,15+,16-,17+,18+/m1/s1 |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-005a-1983000000-2f46d0d29b48132f7670 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000i-4970000000-1aa2bbf3f32fd687c37d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-005a-1983000000-2f46d0d29b48132f7670 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-1390000000-492aa66975c4e026b9d8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-009i-2033900000-46c301a46b654b6c6bf3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4i-5900000000-2102cb581ac98fea87b0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-1900000000-1fab12be883127f80c67 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4i-5900000000-2102cb581ac98fea87b0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (JEOL JMS-01-SG-2) , Positive | splash10-000i-4970000000-1aa2bbf3f32fd687c37d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-000i-0390000000-891430228022fb330b42 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-7ce66600861479086f4f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1970000000-6d5c77ab4f1744109b86 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-331480c31f6585f70cf2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0zg0-0590000000-6ee9bd7dbdbd777f46a4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2900000000-d7ae7d481cc02d1ab839 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2920000000-d7d95b3d24e7be97e848 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-3aa436f48e23664bd455 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-b047d204e97271aa8a65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-0490000000-c0c0bd8b1dc1381c06fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-3950000000-b7180dbe548021fd7fc9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-743915792d6f69593b2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-8469d578b4631e8de995 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02p0-0190000000-738399876b56d0e183d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-843a74fabb1d0eb512a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-0090000000-4e0854fb8be9cf34de97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02br-0090000000-70686fa9219ae093945a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-1126a56061a8c854c755 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fga-0790000000-a1dba0b6195890b30d91 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6v-3910000000-64d9abb8f215c1264e1f | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-000i-3950000000-c2f0781bc5056d66aaf5 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|